Abstract
Objectives
The aim of this study was to determine the role of preepiglottic space (PES) invasion in lymph node metastasis and prognosis in patients undergoing supracricoid partial laryngectomy (SCPL) with cricohyoidopexy (CHP).
Methods
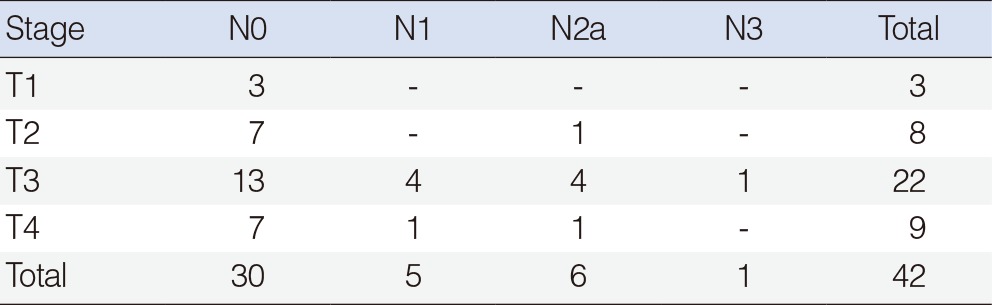
A retrospective review of 42 previously untreated patients with squamous cell carcinoma of the larynx that underwent surgery was performed. The mean age of the subjects was 61.3 years, and the male-to-female ratio was 38:4. Regarding their pathological stages, there were 3, 8, 22, and 9 cases of stage T1 to T4, respectively. Concerning the disease stage of the cervical lymph nodes, there were 30, 5, 6, and 1 cases with N0 to N3, respectively.
Results
The PES invasion rate was 23.8% (10/42). Significant correlations were found between PES invasion and cervical lymph node metastasis (P=0.002). Seven of the 10 patients (70.0%) with PES invasion had cervical lymph node metastasis, whereas only 5 of the 32 patients (15.6%) without any evidence of PES invasion had lymph node metastasis. There was also a significant correlation of PES invasion with age (P=0.002) and T stage (P=0.030). However, there was no significant relationship between gender, primary tumor site, anterior commissure invasion, subglottic extension, paraglottic space invasion and PES invasion. There was a 5-year disease-specific survival of 70%. PES invasion served as a statistically significant prognostic factor for disease-specific survival (P=0.004). Cervical nodal metastasis (P=0.003) and subglottic extension (P=0.01) were also statistically significant prognostic factors associated with disease-specific survival.
Go to : 
Supracricoid partial laryngectomy (SCPL) is a surgical technique used for en bloc resection of the thyroid cartilage, paraglottic space (PGS), and preepiglottic space (PES) with an oncological outcome comparable to a total laryngectomy [1,2]. SCPL is classified according to the type of reconstruction required after resection: cricohyoidoepiglottopexy (CHEP) for glottic carcinomas and cricohyoidopexy (CHP) for tumors with supraglottic components. The SCPL with CHP is an alternative to radiation therapy, supraglottic laryngectomy, and near total and total laryngectomy in selected cases of supraglottic and transglottic carcinoma. The indications for SCPL with CHP are: carcinoma of the supraglottis with involvement of the glottis and anterior commissure; ventricle and PGS invasion; marked limitation of vocal cord mobility; minimal thyroid cartilage invasion; and, glottic carcinoma with involvement of both vocal cords and the anterior commissure [2,3,4]. This procedure is a true organ preservation technique and should be considered as a conservative laryngeal technique since it preserves physiological rehabilitation of speech, swallowing and respiration without a permanent tracheostomy.
The PES was first described in the late 1700s by Boyer, who considered that the region functioned as a prethyrohyoid bursa [5]. It is separated from mucosal surfaces of the head and neck by the hyoepiglottic ligament superiorly, the thyrohyoid membrane anteriorly, and the anterior surface of the epiglottis and thyroepiglottic ligaments posteroinferiorly. These boundaries demarcate a relatively avascular, inverted pyramid-shaped space that contains lymphatics, fat, and loose areolar tissue [6,7]. Invasion of tumor into such a sequestered avascular space signifies advanced disease that may be difficult to eliminate by radiation therapy, which is in part dependent on adequate tissue oxygenation. Supraglottic infrahyoid carcinomas can easily involve the preepiglottic space by direct invasion through multiple fenestrations of the epiglottic cartilage or via the quadrangular membrane [8]. Ljumanovic et al. [9] reported that PES involvement in patients with supraglottic tumors is predictive of local recurrence in patients treated with definitive radiation therapy alone.
The purpose of this study was to analyze the PES invasion yield, and to determine the nature of the relationship between these tumors and cervical nodal metastases and consequent disease specific survival in patients undergoing SCPL with CHP.
Go to : 
The clinical and pathological data from 42 previously untreated patients who were diagnosed with laryngeal carcinoma and underwent SCPL with CHP at the Department of Otolaryngology-Head and Neck Surgery, The Catholic University of Korea, Seoul, Korea, from 1993 to 2010, were reviewed. The choice for SCPL has been dependent on the application of strict oncological selection criteria. SCPL was considered especially in patients with stage T1 tumors that were suspected of involving the anterior commissure or thyroid cartilage, in whom impaired vocal cord mobility was observed, or in whom PGS invasion was suspected in preoperative imaging. All patients had neck dissection at the time of the primary surgery: a radical neck dissection was performed in 2 cases, a modified radical neck dissection in 2 cases, and a selective (levels II-IV) neck dissection in 38 cases. For the 26 contralateral cases, selective (levels II-IV) neck dissection was performed in 25 patients and a modified radical neck dissection in 1. We examined the relationship between PES invasion and clinicopathological factors such as age, gender, tumor stage, anterior commissure invasion, PGS invasion, and subglottic extension. The patients were staged according to the 2002 edition of the tumor, node, metastasis (TNM) classification of the American Joint Committee on Cancer (AJCC). The Institutional Review Board of Seoul St. Mary's Hospital approved this retrospective review of the medical records and the use of archived tumor specimens.
As we previously reported, the resected tissue was fixed in 10% formaldehyde, decalcified in 8% formic acid for 4 days, and embedded in paraffin [10]. The surgical specimens were vertically or horizontally sectioned, depending on the extent of tumor involvement. Vertical section was used for a better representation of the PES, thyroid cartilage, anterior commissure and subglottis. Horizontal cross section allowed for a better visualization of the relationship of the tumor to the PGS. The tissue sections underwent standard histopathologic evaluation. Complete histopathologic review was done in all cases by a single pathologist.
To analyze statistically significant relationships between the distribution of categorical values, the chi-square test, Fisher exact test, multiple logistic regression analysis, multiple linear regression analysis and correlation analysis were used, as appropriate. The survival was determined using the Kaplan-Meier method. The Cox proportional hazard model with likelihood ratio statistics was used to further evaluate each variable by multivariate survival analysis. A P<0.05 was considered statistically significant. All calculations were performed using SPSS ver. 13.0 (SPSS Inc., Chicago, IL, USA).
Go to : 
The mean age of the subjects was 61.3 years (range, 41 to 76 years), and the male-to-female ratio was 38:4. Regarding their pathological stages, there were 3, 8, 22, and 9 cases of stage T1 to T4, respectively. Concerning the disease stage of the cervical lymph nodes, there were 30, 5, 6, and 1 cases with N0 to N3, respectively (Table 1). The tumor originated from the supraglottis and transglottis in 13 and 29 cases, respectively. Postoperative radiotherapy was performed in 8 patients.
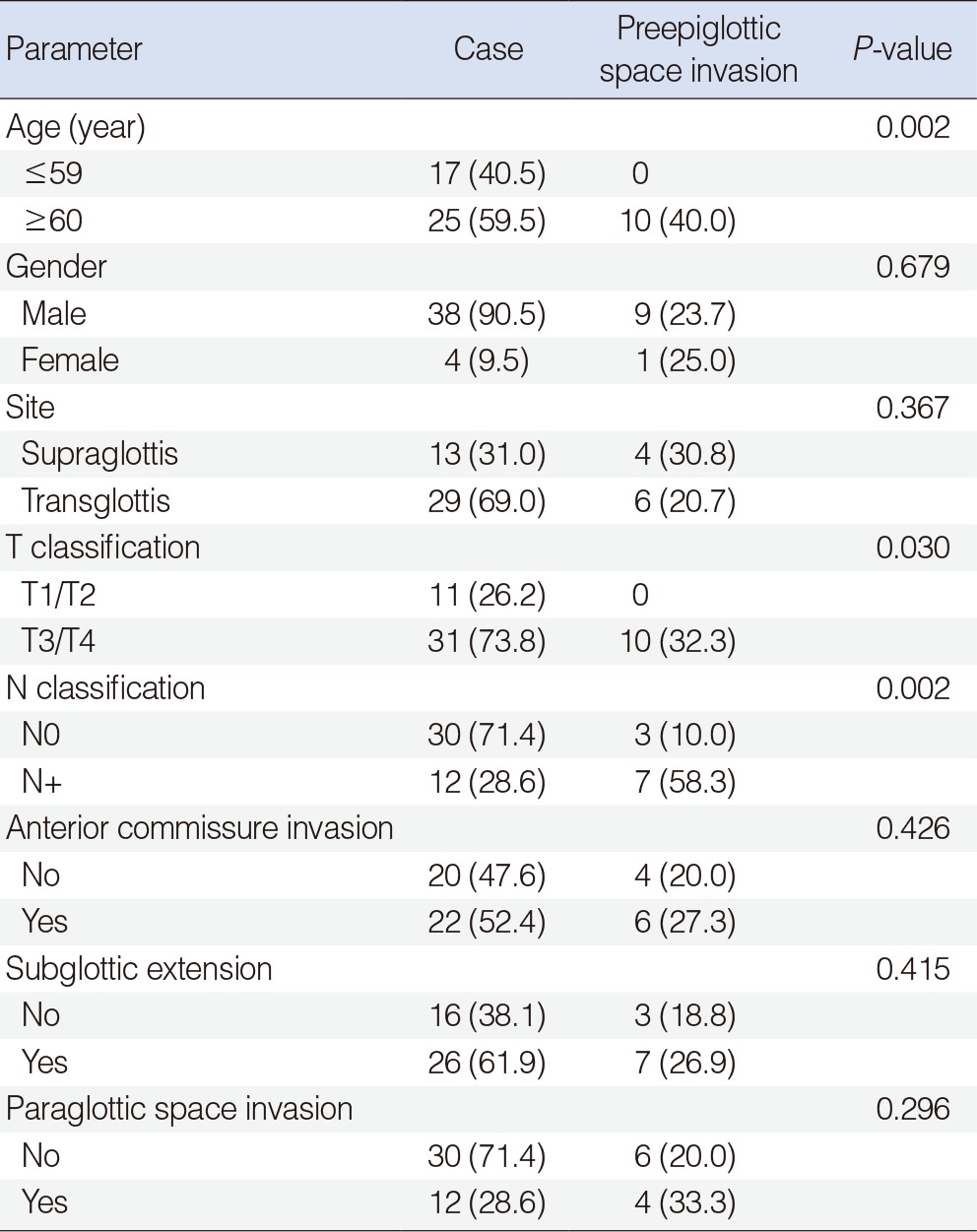
Among the 42 patients undergoing SCPL with CHP, 10 patients (23.8%) developed PES invasion (Table 2). Seven of the 12 patients (58.3%) with cervical lymph node metastasis had PES invasion. In contrast, only 3 of the 30 patients (10.0%) without any evidence of lymph node metastasis had PES invasion. This difference was statistically significant (P=0.002). There was also a significant correlation of PES invasion with age (P=0.002) and T stage (P=0.030). However, there was no significant relationship between gender (P=0.679), primary tumor site (P=0.367), anterior commissure invasion (P=0.426), subglottic extension (P=0.415), PGS invasion (P=0.296) and PES invasion. In a multivariate analysis, there was no clinicopathological factor related to PES invasion.
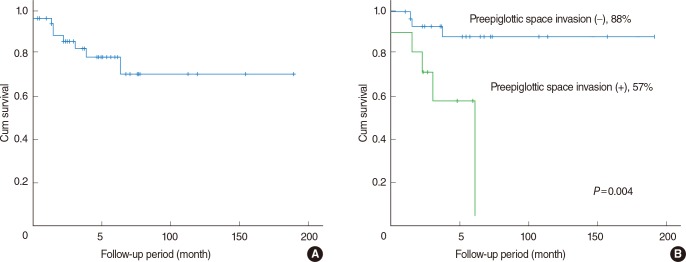
The follow-up period ranged from 2 to 191 months, with a mean of 49 months. Recurrences or metastases occurred in 10 patients and the recurrence rate was 24% (10/42). Three cases were at the primary site, 2 cases were in the neck, 2 cases had distant metastasis, 1 case had both local and regional recurrence, and 2 cases had both regional recurrence and distant metastasis. There was a 5-year disease-specific survival of 70%. The 5-year disease-specific survival among 10 patients with PES invasion was 57%, whereas for the 32 PGS invasion-free patients it was 88% (Fig. 1). This result reached statistical significance (P=0.004). Cervical nodal metastasis (P=0.003) and subglottic extension (P=0.01) were statistically significant prognostic factors associated with disease-specific survival. Age, gender, T stage, margin involvement, postoperative irradiation, anterior commissure invasion, and PGS invasion were not associated with disease-specific survival. The multivariate survival analysis showed that there were no adverse factors affecting disease-specific survival.
Go to : 
The aim of SCPL is to achieve the same local control rates as with total laryngectomy while sparing the function of the larynx. Some authors showed that a probability of 5-year survival rate in patients with SCPL was 92.62% [11]. SCPL is an oncologically safe procedure to preserve laryngeal functions in selected patients with glottic and supraglottic carcinomas [12,13]. Laccourreye et al. [1,2] reported on SCPL as removing the PES, epiglottis and PGS, including thyroid cartilage. Depending on the degree of invasion of the tumor, both of the arytenoids can be spared or one of them can be removed. The reconstruction is immediately carried out by approximation of the cricoid cartilage to the hyoid by CHP, to form a neo-larynx. Thus natural respiration, phonation, deglutition, and sphincteric functions can be maintained without the necessity of a permanent tracheostoma [2,3]. In SCPL with CHP, the thyroid cartilage, the PGS, the PES, and the whole epiglottis are excised, whereas the hyoid bone, the cricoid cartilage, and one or two arytenoid cartilages are preserved.
The PES of the larynx is a loose areolar area composed of elastic and collagenous fibers and adipose tissue. Since the first description by Boyer, several authors have defined its extension [5,14]. The PES is surrounded by the hyoepiglottic ligament superiorly, by the thyrohyoid membrane and thyroid cartilage anteriorly, and by the thyroepiglottic ligament and epiglottic cartilage posteroinferiorly [14]. Overall, PES involvement is a relatively common occurrence in advanced squamous cell carcinoma of the head and neck, particularly in tumors arising from the supraglottis and the base of tongue. The PES is frequently involved with tumor, probably because the elastic epiglottis cartilage has anatomic perforations and is easily penetrated below the hyoid bone, which allows direct extension of infrahyoid supraglottic cancer into this fascia-bound space [15]. In a histopathologic study of 80 resected larynges, Gregor [16] reported that PES invasion was present in 62.5% of cases. Pathologic evaluation of surgical specimens has shown a high incidence of PES invasion (T3) in patients with supraglottic carcinomas clinically staged as TI or T2 [6,17]. The PES invasion is more common with supraglottic carcinomas occurring below the level of the hyoid bone than for those suprahyoid in location (15% and 6%, respectively) [12,18]. Ljumanovic et al. [9] found, in a retrospective series of 84 patients with supraglottic tumors, that magnetic resonance imaging evidence of PES invasion was statistically associated with local recurrence in patients treated with definitive radiation therapy alone. Fletcher and Hamberger [19] suggested that local failure of radiation treatment of tumors involving the PES is due to the relatively avascular nature of this anatomical compartment. The supraglottic part of the larynx is characterized by a rich lymphatic network and carcinomas of this region have a strong predilection for cervical metastasis. Werner et al. [20] investigated the architecture and drainage patterns of the lymphatic system of the upper aerodigestive tract in 850 organ specimens using microscopic techniques combined with in vivo and in vitro lymphographic studies. They found that the lymph fluid of the supraglottic space drains through the lateral part of the thyrohyoid membrane (anterior boundary of the PES) in a mediolateral direction over three to six lymphatic channels.
In our study, the overall PES invasion rate was 23.8%. The patients with PES invasion had a significantly higher rate of cervical lymph node metastasis compared to patients without PES invasion. Patients with no evidence of PES invasion may have a survival benefit. The rate of 5-year disease-specific survival decreased dramatically in the presence of PES invasion and cervical lymph node metastasis using univariate analysis, but did not reach significance by multivariate analysis.
There were some limitations in this study. The main limitation was the retrospective aspect of the analysis. The second limitation was the small number of patients undergoing SCPL with CHP. Lastly, the median follow-up time of this study was rather short, which might lead to limitations of the disease-specific survival data. Further studies with a larger number of subjects should be our finding that PES invasion influences prognosis.
In conclusion, PES invasion was found to be significantly related to the presence of cervical lymph node metastasis and prognosis in patients undergoing SCPL with CHP. Therefore, PES invasion is a valuable marker that might be used to help guide the aggressiveness of therapy in future clinical trials and/or practice.
Go to : 
References
1. Laccourreye H, Laccourreye O, Weinstein G, Menard M, Brasnu D. Supracricoid laryngectomy with cricohyoidoepiglottopexy: a partial laryngeal procedure for glottic carcinoma. Ann Otol Rhinol Laryngol. 1990; 6. 99(6 Pt 1):421–426. PMID: 2350125.

2. Laccourreye H, Laccourreye O, Weinstein G, Menard M, Brasnu D. Supracricoid laryngectomy with cricohyoidopexy: a partial laryngeal procedure for selected supraglottic and transglottic carcinomas. Laryngoscope. 1990; 7. 100(7):735–741. PMID: 2362533.
3. Brasnu D, Menard M, Fabre A, Janot F, Laccourreye H. Partial supracricoid laryngectomies: techniques, indications and results. J Otolaryngol. 1988; 6. 17(4):173–178. PMID: 3398106.
4. Levine PA, Brasnu DF, Ruparelia A, Laccourreye O. Management of advanced-stage laryngeal cancer. Otolaryngol Clin North Am. 1997; 2. 30(1):101–112. PMID: 8995139.

5. Lee WT, Rizzi M, Scharpf J, Lorenz RR, Saxton JP, Adelstein DJ, et al. Impact of preepiglottic space tumor involvement on concurrent chemoradiation therapy. Am J Otolaryngol. 2010; May-Jun. 31(3):185–188. PMID: 20015743.

6. Zeitels SM, Vaughan CW. Preepiglottic space invasion in "early" epiglottic cancer. Ann Otol Rhinol Laryngol. 1991; 10. 100(10):789–792. PMID: 1952643.

7. Pogura JH. Surgical pathology of cancer of the larnyx. Laryngoscope. 1955; 10. 65(10):867–926. PMID: 13264674.
8. Dursun G, Keser R, Akturk T, Akiner MN, Demireller A, Sak SD. The significance of pre-epiglottic space invasion in supraglottic laryngeal carcinomas. Eur Arch Otorhinolaryngol. 1997; 254(Suppl 1):S110–S112. PMID: 9065642.

9. Ljumanovic R, Langendijk JA, Schenk B, Van Wattingen M, Knol DL, Leemans CR, et al. Supraglottic carcinoma treated with curative radiation therapy: identification of prognostic groups with MR imaging. Radiology. 2004; 8. 232(2):440–448. PMID: 15286316.

10. Sun DI, Cho KJ, Cho JH, Joo YH, Jung CK, Kim MS. Pathological validation of supracricoid partial laryngectomy in laryngeal cancer. Clin Otolaryngol. 2009; 4. 34(2):132–139. PMID: 19413611.

11. Szyfter W, Leszczynska M, Wierzbicka M. Outcome after supracricoid laryngectomies in the material of ENT Department, Poznan University of Medical Sciences. Eur Arch Otorhinolaryngol. 2011; 6. 268(6):879–883. PMID: 21305310.

12. Cho KJ, Joo YH, Sun DI, Kim MS. Supracricoid laryngectomy: oncologic validity and functional safety. Eur Arch Otorhinolaryngol. 2010; 12. 267(12):1919–1925. PMID: 20490818.

13. Sanchez-Cuadrado I, Castro A, Bernaldez R, Del Palacio A, Gavilan J. Oncologic outcomes after supracricoid partial laryngectomy. Otolaryngol Head Neck Surg. 2011; 6. 144(6):910–914. PMID: 21493316.

14. Sato K, Kurita S, Hirano M. Location of the preepiglottic space and its relationship to the paraglottic space. Ann Otol Rhinol Laryngol. 1993; 12. 102(12):930–934. PMID: 8285513.

15. Kirchner JA, Som ML. Clinical and histological observations on supraglottic cancer. Ann Otol Rhinol Laryngol. 1971; 10. 80(5):638–645. PMID: 18478663.

16. Gregor RT. The preepiglottic space revisited: is it significant? Am J Otolaryngol. 1990; May-Jun. 11(3):161–164. PMID: 2382783.
17. Zeitels SM, Vaughan CW, Domanowski GF. Endoscopic management of early supraglottic cancer. Ann Otol Rhinol Laryngol. 1990; 12. 99(12):951–956. PMID: 2244727.

18. Petrovic Z, Krejovic B, Djukic V, Stankovic P. Primary surgical treatment for carcinoma of the larynx: influence of the local invasion. J Laryngol Otol. 1991; 5. 105(5):353–355. PMID: 2040837.
19. Fletcher GH, Hamberger AD. Causes of failure in irradiation of squamous-cell carcinoma of the supraglottic larynx. Radiology. 1974; 6. 111(3):697–700. PMID: 4829005.

20. Werner JA, Dunne AA, Myers JN. Functional anatomy of the lymphatic drainage system of the upper aerodigestive tract and its role in metastasis of squamous cell carcinoma. Head Neck. 2003; 4. 25(4):322–332. PMID: 12658737.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download