Abstract
Objective
This study was conducted to present the clinical outcome of invasive fungal sinusitis of the sphenoid sinus and to analyze clinical factors influencing patient survival.
Results
Cases were divided into acute fulminant invasive fungal spheonoidits (n=4) and chronic invasive fungal sphenoiditis (n=8). The most common underlying disease was diabetes mellitus (n=9). The most common presenting symptoms and signs included visual disturbance (100%). Intracranial extension was observed in 8 patients. Endoscopic debridement and intravenous antifungals were given to all patients. Fatal aneurysmal rupture of the internal carotid artery occurred suddenly in two patients. The mortality rate was 100% for patients with acute fulminant invasive fungal sphenoiditis and 25% for patients with chronic invasive fungal sphenoiditis. In survival analysis, intracranial extension was evaluated as a statistically significant factor (P=0.027).
Conclusion
The survival rate of chronic invasive fungal sphenoiditis was 75%. However, the prognosis of acute fulminant invasive fungal sphenoiditis was extremely poor despite the application of aggressive treatment, thus, a high index of suspicion should be required and new diagnostic markers need to be developed for early diagnosis of invasive fungal sinusitis of the sphenoid sinus.
Fungal sinusitis is generally classified into allergic, chronic non-invasive (fungus ball), chronic invasive, granulomatous invasive, and acute fulminant invasive fungal sinusitis based on histological features according to the diagnostic criteria of deShazo et al. [1,2]. In addition to these, other types such as saprophytic colonization [3] and semi-invasive fungal infection [4] have been suggested in the literature. The incidence of invasive fungal sinusitis (IFS) has been increasing due to the increasing number of immunosuppressive patients with diabetes, hematologic malignancies and prolonged use of steroids. IFS can also occur in immunocompetent hosts [5,6,7,8].
In contrast to the non-invasive type which usually has a good prognosis, IFS is considered a potentially lethal condition. Moreover, invasive fungal sphenoiditis is more aggressive than invasive fungal infection of the other paranasal sinuses. This is due to the involvement of important surrounding structures such as the orbital apex, cavernous sinus, optic nerve, internal carotid artery, pituitary gland, and cranial nerves. Patients with early stage sphenoid sinus lesions are usually asymptomatic, thus, the diagnosis is often delayed until they are presented to ear, nose and throat specialists. Because advanced invasive fungal infection of the sphenoid sinus carries significant mortality, early diagnosis and appropriate treatment are crucial for the improvement of patient survival. In this study, we report the clinical features and treatment outcomes of IFS of the sphenoid sinus. In addition, this study also analyzed clinical factors influencing patient survival.
Medical records of 12 cases of IFS of the sphenoid sinus registered between 2001 and 2010 at the Chonnam National University Hospital and Hwasun Hospital were reviewed. A retrospective review was conducted to evaluate the underlying diseases, clinical manifestations, radiologic findings, treatment, and prognosis of patients with IFS of the sphenoid sinus. The follow-up period ranged from 12 days to 55 months with a median of 24 months.
Patients with IFS of the sphenoid were included if the lesion in the sphenoid sinus was exclusively involved, or if the main lesion was located in the sphenoid sinus and extended outside the sphenoid sinus into the posterior ethmoid, orbital apex, superior orbital fissure, pterygopalatine fossa, dura, cavernous sinus, and/or cerebrum. Diagnosis of IFS of the sphenoid sinus was made according to clinical findings such as cranial nerve involvement and the presence of underlying diseases such as diabetes, hematologic disorder, etc. and/or histologic evaluation of the sphenoid sinus mucosa revealing the characteristic morphology of fungi such as Aspergillus or Mucor species. According to Chakrabarti et al. [9], differentiation between the acute IFS and the chronic IFS was done based on the speed of progression of the clinical course; acute IFS refers to disease <4 weeks in duration in immunocompromised hosts, and chronic IFS is defined as locally invasive disease over the duration of at least 3 months.
All patients underwent surgery using an endoscopic transethmoidal approach, and one patient underwent combined external surgery with neurosurgeons. Sphenoidotomy opening was enlarged as widely as possible after which the sphenoid sinus was inspected. If there was a fungus ball and mucosal swelling, removal of the fungus ball and conservative debridement, including multiple biopsies, were performed. On the other hand, if necrosis was observed in the mucosa with suspected IFS, multiple biopsies were performed. After making a definite diagnosis of IFS, aggressive surgery including extensive debridement was performed: lesions extending to the sites beyond the sphenoid sinus, such as the orbital apex and pterygopalatine fossa, were removed through the medial wall of the orbital apex or the posterior wall of the maxillary sinus (Fig. 1).
Clinical parameters with the potential to influence overall survival were divided into two groups for univariate analysis: diabetes mellitus (DM) control (good vs. poor), the status of the mucosa (necrosis vs. swelling), involvement of cranial nerves (multiple vs. the optic nerve only), bone destruction of the sphenoid sinus (present vs. absent), interval between the onset of clinical symptoms and initiation of treatment, types of debridement (limited vs. extensive), and intracranial extension (yes vs. no). The time period between initiation of treatment and death was analyzed by the Kaplan-Meier method, and survival difference between the two groups were compared with the univariate log-rank test. Statistical analysis was performed using SPSS ver. 14.0 (SPSS Inc., Chicago, IL, USA), and statistical significance was defined as P<0.05.
Twelve patients (4 males and 8 females) were included in the study. The age of the patients ranged from 57 to 76 years, with a mean of 68.8±5.1 years. The most common underlying disease was DM in 9 patients; other associated diseases included chronic renal failure, multiple myeloma, and hypertension. Diagnosis was based on the histologic identification of the fungus, including Aspergillus species (10 cases) and Mucor species (2 cases). Fungal culture was negative in all cases due to low sensitivity. Of the 12 patients, acute fulminant invasive fungal sphenoiditis was observed in 4 cases and chronic invasive fungal sphenoiditis in 8 cases. Clinical manifestation was strongly suggestive of IFS, but tissue invasion was not noted in biopsy in 4 cases. In such cases, they were diagnosed as 'possible IFS,' according to the standard definition of invasive fungal infection [10]. Two cases of invasive fungal sphenoiditis occurred in immunocompetent hosts (Tables 1, 2).
Clinical manifestations included visual disturbance (100%), headache (8/12, 67%), and ophthalmoplegia (8/12, 67%), followed by ptosis, facial pain, and orbital pain. The location of headache was variable, including diffuse, retro-orbital, and frontal regions. Multiple cranial nerve involvement was observed in 9 cases, but isolated involvement of the optic nerve was noted in 3 cases. All patients had visual disturbance, including decreased visual acuity and diplopia. Interval from the onset of symptoms to initiation of treatment ranged from 1 week to 1 year. Patients with visual disturbance presented early, but patients with headache or facial pain presented late because of its vague nature.
Computed tomography (CT) scan was performed as an initial imaging study in all patients (Figs. 2, 3). Calcification within the sphenoid sinus on CT was not noted in all patients. The most common CT finding was opacification of the sphenoid sinus (8 cases with total opacification and 4 cases with partial opacification), followed by bony destruction (n=9) and sclerotic thickening (n=7). Destruction of the sphenoid sinus wall included no destruction (n=3), focal destruction (n=4), and diffuse destruction (n=5). The locations of destruction of the sphenoid sinus were the superior wall (n=1) and lateral wall (n=8). MRI was superior to CT in detecting the involvement of extra-sphenoidal lesions. Sphenoid lesions showed variable intensities according to the presence of fungus balls and surrounding mucosal status. Extra-sphenoid sinus lesions revealed iso-signal intensity on T1WI and low- or iso-signal intensity on T2WI in most of the cases. Gd-enhancement T1WI uniformly showed enhancement of lesions. Unilateral involvement of the sphenoid sinus was noted in 11 cases except for one case with bilateral involvement. Extra-sphenoidal involvement was noted in all 12 patients. Intracranial extension was observed in 8 of 12 patients, and the involved sites were the cavernous sinus, cerebrum, meninges, and internal carotid artery (n=8).
Surgical debridement was undertaken through an endoscopic approach, except for one patient whose lesion had extended to the frontal lobe, and was treated with a combined endoscopic and external approach by craniotomy. Limited debridement was done for sphenoid lesions to avoid the potential risk of the internal carotid artery or cavernous sinus, and extensive debridement was applied to extra-sphenoidal lesions including the orbit, pterygopalatine fossa and infratemporal fossa. Exploration of the orbit, pterygopalatine fossa, and/or infratemporal fossa was performed through a transethmodial approach. Multiple surgeries (2 to 3 times) were done in 4 cases. On surgical findings, fungus balls were noted in 9 of 12 cases (75%), and mucosal necrosis was present in 6 of 12 cases. The presence of fungus ball in invasive fungal sphenoiditis suggests the potential of a non-invasive fungal ball to be transformed into IFS in immunocompromised conditions.
Antifungal treatment included various combinations of amphotericin, itraconazole, and voriconazole. Amphotericin was an initial antifungal drug used in 11 cases except for one case in which voriconazole was initially given due to the patient's poor renal function. A total accumulative dose of 2.0 g amphotericin was usually administered. Toxicities due to amphotericin occurred in 4/11 cases in which amphotericin was changed to liposomal amphotericin or voriconazole. After cessation of intravenous antifungal therapy, medication with itraconazole or voriconazole was additionally administered for 3 months or longer according to the patient's status. Adjuvant hyperbaric oxygen therapy was applied to a patient who had extensive involvement in the orbital apex, pterygopalatine fossa, and middle cranial fossa.
Improvement of visual acuity and extraocular motion limitation was seen in 3 of 12 cases (25%) and in 2 of 12 cases (17%), respectively. The overall survival rate was 50% (6/12); the survival rate of acute fulminant fungal sphenoiditis and chronic invasive fungal sphenoiditis was 0% and 75%, respectively. All six dead patients had extension into the intracranial region. Interestingly, two patients developed unexpected subarachnoidal hemorrhage due to aneurysmal rupture of the internal carotid artery at postoperative day #5 and #14 and eventually died. Other causes of death included cerebral infarction, acute renal failure, and shock.
Among multiple factors affecting patient survival time, intracranial extension was the only statistically significant factor (P=0.027). Other clinical parameters, including mucosal necrosis of the sphenoid sinus, showed no statistical significance associated with patient survival (Table 3).
The typical symptoms of IFS of the sphenoid sinus are prolonged diffuse headache and rhinorrhea, followed by sudden visual disturbance or ocular motion impairment resulting from orbital apex syndrome or cavernous sinus syndrome [11]. In our study, the most common associated symptoms and signs were visual disturbance and headache. All patients presented with ocular symptoms, including decreased visual acuity or diplopia. These patients often had orbital apex syndrome or less frequently, a more severe form, cavernous sinus syndrome. Although headache was sometimes preceded by ocular symptoms in our patients, the possibility of sphenoid lesions was not suspected at that time, which resulted in delayed diagnosis. Although headache in sphenoid lesions is deep seated and retro-orbital [8], it has been generally accepted that headache is a nonspecific symptom and the location of the headache generally do not suggest the presence of a sphenoid lesion [12,13]. Similarly, the distribution of headache was variable in our case study, and headache locations were not specific for sphenoid sinus lesions. Nevertheless, a high index of suspicion is required in cases with mildly immunosuppressive patients such as patients with DM complaining of headache or decreased visual acuity. Loss of vision was due to the optic nerve involvement. Diplopia was subjective or more commonly secondary to ophthalmoplegia due to multiple nerve palsy. Our patients did not have an isolated sixth nerve palsy which is one of the earliest signs of a sphenoid sinus lesion. This may suggest that IFS is likely to have extensive neural involvement. All our cases showed extension to the optic nerve and/or orbital apex, and 6 patients showed involvement of the pterygopalatine fossa, skull base, or brain at presentation, suggesting invasive nature and delayed diagnosis of the disease. In the early stage of IFS, the nasal mucosa appears pale. In particular, the central hidden location of the sphenoid sinus make rigid endoscopic examination more difficult and thus flexible endoscopy is deemed more desirable after vasoconstriction of the sinonasal mucosa. Early endoscopic evaluation with biopsy of healthy and diseased tissues and cultures of sinus contents is required in cases of suspected IFS [14,15].
CT is the most commonly used imaging study for suspected IFS because of its superiority in detecting bone destruction. Although bone destruction is one of the radiologic findings of IFS, these fungi such as Mucor tend to extend along the vessels, and extension beyond the sinuses may occur with intact bony sinus walls [16]. In our case series, focal or diffuse bone destruction was observed in 9 of 12 cases. Conversely, sclerotic changes in the bony walls of the sphenoid sinus were noted in 7 cases, representing chronic sinus disease with or without fungus balls. Moreover, patients with IFS generally do not have bone erosion or extension of the disease outside of the sinuses and unilateral mucosal thickening on CT in the early stage. Opacification of the sphenoid sinus was the most common finding. However, mucosal thickening in the early stage may sometimes be very subtle and nonspecific in radiological studies. Calcification suggesting fungus ball on CT was not seen in all cases, although fungus balls were identified in 9 cases intraoperatively. An invasive fungal lesion may appear to be a mass and masquerade as a malignancy with destruction of the sinus walls and extension beyond the sinus confines [16]. Magnetic resonance imaging (MRI) is superior to CT scan in evaluating intracranial and intraorbital extension beyond the sphenoid sinus, as shown in our study. Enhanced ill-defined lesions in the orbit, cavernous sinus, and/or brain were seen in all cases. Groppo et al. [17] reported that MRI was more sensitive in detecting early changes of acute fulminant IFS than CT, and perisinus invasion on MRI was the most sensitive and specific parameter.
However, current diagnostic methods including imaging studies are not sufficiently sensitive or specific, and results are often available too late to be clinically useful. Early diagnosis and/or treatment has been shown to improve patient outcome. Vague symptoms of sphenoid lesions lead to delayed presentation. Time to presentation in our study ranged from 1 week to 1 year. New diagnostic markers, including Aspergillus galactomanan (GM) and β-glucan, are available along with polymerase chain reaction based assay in high risk patients with neutropenia or bone marrow transplant.
The mainstays of treatment are aggressive surgical debridement and antifungal agents. Early aggressive sinonasal debridement should be performed in all patients with IFS. However, the sphenoid sinus wall comprises important neurovascular structures, thus often limiting the use of radical debridement. When the orbital apex is involved, the decision regarding orbital exenteration remains extremely difficult. However, orbital exenteration does not guarantee a cure of IFS. Dhiwakar et al. [18] reported that orbital exenteration appears to be justified for posterior orbital (retrobulbar, apical) diseases regardless of the functional status of the eye, but is not appropriate for anterior orbital (inferomedial) diseases. Although all our cases involved the optic nerve and/or orbital apex, orbital exenteration was not attempted. We removed the necrotic tissue in the sphenoid sinus, but left the sinus wall undisturbed to avoid inadvertent injury to the internal carotid artery, cavernous sinus, or cranial nerves. Instead, lesions in the orbital apex, infratemporal fossa, or skull base was endoscopically explored and removed. Univariate analysis showed that the extent of debridement was not a significant factor of patient survival. Nevertheless, we believe that aggressive globe preserving debridement is effective in controlling early lesions and is a feasible alternative to eyeball exenteration.
Radical removal of diseased tissue is often impossible and other treatments, including antifungals, should be added to control microscopic or residual diseases. Antifungals include triazole (iatraconazole, voriconazole, posaconazole), polyenes (amphotercin and its lipid formulations), and other class agents (echinocandins and flucytosine). Amphotericin B has a broad spectrum of activity against Candida spp., Aspergillus spp., Cryptococcus spp., Fusarium spp., Mucorales, and endemic fungi [19,20]. Triazoles are the only fungicides against Aspergillus spp., and echinocandins against Candida [20]. In contrast to other triazoles, posaconazole has activity against Zygomycetes including Mucor spp., Rhizopus spp., and Cunninghamella spp. The lipid complex and liposomal form of amphotericin are alternative drugs used in cases with impaired renal function. Recently, retrobulbar amphotericin injection has been used for orbital aspergillosis [21,22]. Hyperbaric oxygen therapy has been sporadically used in IFS [23,24]. In our study, hyperbaric oxygen therapy was used in a patient with involvement of the orbit and cerebrum, who, later, died of intracranial extension. We found that the deep tissue had progressed to necrosis, although the superficial tissue of the involved area looked relatively healthy with addition of hyperbaric oxygen therapy. Therefore, controlled randomized studies with regard to hyperbaric oxygenation need to be conducted. Moreover, the patient's immunosuppressive state should be corrected. If a patient is neutropenic or diabetic, GM-cerebrospinal fluid should be given, or blood sugar should be strictly controlled.
Visual outcome of IFS involving the orbit is poor. In our study, improvement in visual acuity and extraocular motion limitation was achieved in 27% (3/11) and 25% (2/8), respectively. Choi et al. [25] reported that permanent loss of light perception in the affected eye occurred in 12 of 15 patients (80%), who had no visual loss prior to infection and showed worsening vision after infection.
The overall survival rate was 50% in our study. Despite the extremely poor outcome of acute fulminant invasive fungal sphenoiditis, the treatment outcome of chronic invasive fungal sphenoiditis in our study (survival rate of 75%) was comparable with other studies reporting survival rates of 60% and 100% with limitations such as case reports and short follow-up period [8,11]. Fatal mycotic aneurysmal rupture occurred in two patients. Although treatment of mycotic aneurysms is controversial, endovascular treatment is preferred to an open approach at present [26,27]. In our survival analysis, intracranial extension (P=0.027) was the only significant factor which represented dismal prognosis. Although the mucosal status of the sphenoid (P=0.052) had borderline statistical significance, it should be kept in mind that even a mucosal lesion without necrosis can lead to a fatal course. As shown in two cases with sudden death due to ruptured intracranial mycotic aneurysm in this study, physicians should be aware of the potential of a rapid fatal course and the unpredictability of IFS. Although correction of the immunocompromised state has been well known as an independent factor affecting survival in IFS, DM control was not statistically significant in our study, which may suggest that the involvement of the sphenoid sinus may be more critical than the control of underlying diseases. Also, this finding may be extrapolated from two patients in our study who died in spite of their immunocompetent status. Chopra et al. [8] reported a survival rate of 60% (3/5) in 5 immunocompetent cases with invasive fungal sphenoiditis, despite the relatively short follow-up period of their study.
Our study has some limitations. First, this disease entity is very rare and this study was too small to reach a statistically significant conclusion. Large studies are warranted to validate our results. Second, some cases without evidence of fungal invasion on histopathology were clinically diagnosed as possible invasive fungal sphenoidits. In sphenoid lesions, sampling errors on biopsies are not uncommon due to the risk of damage to major neurovascular structures such as the optic nerve or internal carotid artery. If invasive fungal infection is clinically suspected despite negative biopsy, patients should be treated aggressively as the diagnosis of 'possible invasive fungal sphenoiditis.'
Our results showed that the prognosis of invasive fungal sphenoiditis is extremely poor despite aggressive treatment, which emphasizes the importance of early diagnosis and appropriate treatment. Because invasive fungal sphenoiditis can occur even in immunocompetent patients, a high index of suspicion should be maintained for early diagnosis. New diagnostic tests such as serum GM and β-glucan can be useful as screening tests, especially in immunocompromised patients with vague symptoms of sphenoid sinus lesions such as headache, visual symptoms and nasal symptoms. In addition to correcting the immunosuppressive state, new treatment strategies including new antifungal agents need to be developed.
References
1. deShazo RD, O'Brien M, Chapin K, Soto-Aguilar M, Gardner L, Swain R. A new classification and diagnostic criteria for invasive fungal sinusitis. Arch Otolaryngol Head Neck Surg. 1997; 11. 123(11):1181–1188. PMID: 9366697.

2. deShazo RD, Chapin K, Swain RE. Fungal sinusitis. N Engl J Med. 1997; 7. 337(4):254–259. PMID: 9227932.

3. Ferguson BJ. Definitions of fungal rhinosinusitis. Otolaryngol Clin North Am. 2000; 4. 33(2):227–235. PMID: 10736401.

4. Rowe-Jones JM, Moore-Gillon V. Destructive noninvasive paranasal sinus aspergillosis: component of a spectrum of disease. J Otolaryngol. 1994; 4. 23(2):92–96. PMID: 8028079.
5. Chopra H, Dua K, Bhatia S, Dua N, Mittal V. Invasive rhino-orbital fungal sinusitis following dental manipulation. Mycoses. 2009; 7. 52(4):368–371. PMID: 18705660.

6. Macedo DP, Neves RP, Fontan J, Souza-Motta CM, Lima D. A case of invasive rhinosinusitis by Fusarium verticillioides (Saccardo) Nirenberg in an apparently immunocompetent patient. Med Mycol. 2008; 8. 46(5):499–503. PMID: 18608897.
7. Suryanarayan Rao S, Panda NK, Pragache G, Chakrabarti A, Saravanan K. Sinoorbital mucormycosis due to Apophysomyces elegans in immunocompetent individuals: an increasing trend. Am J Otolaryngol. 2006; Sep-Oct. 27(5):366–369. PMID: 16935188.
8. Chopra H, Dua K, Malhotra V, Gupta RP, Puri H. Invasive fungal sinusitis of isolated sphenoid sinus in immunocompetent subjects. Mycoses. 2006; 1. 49(1):30–36. PMID: 16367816.

9. Chakrabarti A, Denning DW, Ferguson BJ, Ponikau J, Buzina W, Kita H, et al. Fungal rhinosinusitis: a categorization and definitional sche-ma addressing current controversies. Laryngoscope. 2009; 9. 119(9):1809–1818. PMID: 19544383.
10. De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008; 6. 46(12):1813–1821. PMID: 18462102.

11. Baumann A, Zimmerli S, Hausler R, Caversaccio M. Invasive sphenoidal aspergillosis: successful treatment with sphenoidotomy and voriconazole. ORL J Otorhinolaryngol Relat Spec. 2007; 69(2):121–126. PMID: 17159376.

12. Gilony D, Talmi YP, Bedrin L, Ben-Shosan Y, Kronenberg J. The clinical behavior of isolated sphenoid sinusitis. Otolaryngol Head Neck Surg. 2007; 4. 136(4):610–615. PMID: 17418260.

13. An YH, Venkatraman G, DelGaudio JM. Isolated inflammatory sphenoid sinus disease: a revisitation of computed tomography indications based on presenting findings. Am J Rhinol. 2005; Nov-Dec. 19(6):627–632. PMID: 16402654.

14. Blitzer A, Lawson W, Meyers BR, Biller HF. Patient survival factors in paranasal sinus mucormycosis. Laryngoscope. 1980; 4. 90(4):635–648. PMID: 7359982.

15. Loftus BC. General principles of management of fungal infections of the head and neck. Otolaryngol Clin North Am. 1993; 12. 26(6):1115–1121. PMID: 8290284.

16. Aribandi M, McCoy VA, Bazan C 3rd. Imaging features of invasive and noninvasive fungal sinusitis: a review. Radiographics. 2007; Sep-Oct. 27(5):1283–1296. PMID: 17848691.

17. Groppo ER, El-Sayed IH, Aiken AH, Glastonbury CM. Computed tomography and magnetic resonance imaging characteristics of acute invasive fungal sinusitis. Arch Otolaryngol Head Neck Surg. 2011; 10. 137(10):1005–1010. PMID: 22006778.

18. Dhiwakar M, Thakar A, Bahadur S. Invasive sino-orbital aspergillosis: surgical decisions and dilemmas. J Laryngol Otol. 2003; 4. 117(4):280–285. PMID: 12816217.

19. Leventakos K, Lewis RE, Kontoyiannis DP. Fungal infections in leukemia patients: how do we prevent and treat them? Clin Infect Dis. 2010; 2. 50(3):405–415. PMID: 20047485.

20. Vallejo C, Barberán J. Empirical antifungal treatment: a valid alternative for invasive fungal infection. Rev Esp Quimioter. 2011; 9. 24(3):117–122. PMID: 21947092.
21. Mainville N, Jordan DR. Orbital apergillosis treated with retrobulbar amphotericin B. Orbit. 2012; 2. 31(1):15–17. PMID: 22029690.
22. Wakabayashi T, Oda H, Kinoshita N, Ogasawara A, Fujishiro Y, Kawanabe W. Retrobulbar amphotericin B injections for treatment of invasive sino-orbital aspergillosis. Jpn J Ophthalmol. 2007; Jul-Aug. 51(4):309–311. PMID: 17660997.

23. Segal E, Menhusen MJ, Shawn S. Hyperbaric oxygen in the treatment of invasive fungal infections: a single-center experience. Isr Med Assoc J. 2007; 5. 9(5):355–357. PMID: 17591371.
24. Knipping S, Holzhausen HJ, Koesling S, Bloching M. Invasive aspergillosis of the paranasal sinuses and the skull base. Eur Arch Otorhinolaryngol. 2007; 10. 264(10):1163–1169. PMID: 17534639.

25. Choi HS, Choi JY, Yoon JS, Kim SJ, Lee SY. Clinical characteristics and prognosis of orbital invasive aspergillosis. Ophthal Plast Reconstr Surg. 2008; Nov-Dec. 24(6):454–459.

26. Jao SY, Weng HH, Wong HF, Wang WH, Tsai YH. Successful endovascular treatment of intractable epistaxis due to ruptured internal carotid artery pseudoaneurysm secondary to invasive fungal sinusitis. Head Neck. 2011; 3. 33(3):437–440. PMID: 19953634.

27. Hot A, Mazighi M, Lecuit M, Poiree S, Viard JP, Loulergue P, et al. Fungal internal carotid artery aneurysms: successful embolization of an Aspergillus-associated case and review. Clin Infect Dis. 2007; 12. 45(12):e156–e161. PMID: 18190310.

Fig. 1
Endoscopic finding shows a necrotic lesion of the orbital apex and sphenoid sinus (A) and relatively healthy neurovascular structures following debridement of the orbital apex (B). ON, optic nerve; ICA, internal carotid artery; SF, sellar floor.
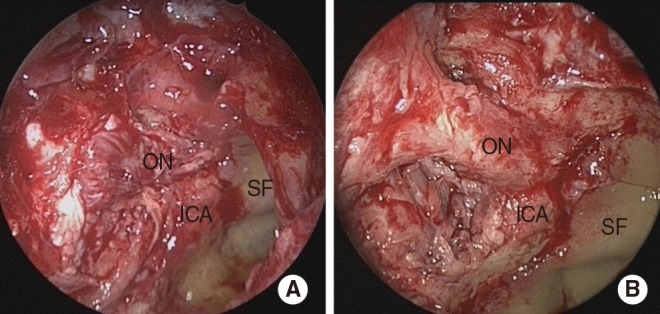
Fig. 2
Acute fulminant fungal sphenoiditis in a 70-year-old female. Coronal computed tomography (A) and magnetic resonance imaging (B) scan show fungal sphenoid sinusitis with lesion extension into the orbital apex and both frontal lobes.

Fig. 3
Chronic invasive fungal sphenoiditis in a 69-year-old male. Coronal computed tomography (A) and magnetic resonance imaging (B) scan show fungal sphenoid sinusitis with lesion extension into the orbital apex, pterygopalatine fossa, cavernous sinus, nasopharynx, and parapharyngeal space.

Table 2
Clinical summary of our cases with IFS of the sphenoid sinus (n=12)

IFS, invasive fungal sinusitis; FU, follow-up; Asp, apsergillus; DM, diabetes mellitus; HTN, hypertension; VD, visual disturbance; OA, oribital apex; TES, transethmoid surgery; PPF, pterygopalatine fossa; explo, exploration; A, amphotericin; VC, voriconazole; CS, cavernous sinus; PPX, parapharynx; MS, masticator space; FR, foramen rotundum; FO, foramen ovale; MCF, middle cranial fossa; IC, itraconazole; HBO, hyperbaric oxygen therapy; CRF, chronic renal failure; ON, optic nerve; ICA, internal carotid artery; MM, multiple myeloma.




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download