This article has been
cited by other articles in ScienceCentral.
Abstract
Objectives
Pregabalin is used to treat neuropathic pain and has shown analgesic properties in postoperative pain. The aim of this study was to investigate the effectiveness and safety of pregabalin in reducing postoperative pain in patients after septoplasty.
Methods
Forty-seven patients scheduled for elective septoplasty were randomly assigned to groups that received either pregabalin (150 mg) or placebo, both one hour before surgery and 12 hours after the initial dose. Pain (verbal numerical rating scale, VNRS) and side effect assessments were performed at 6, 12, 12 to 24, and 24 to 48 hours postoperatively.
Results
From 1 to 12 hours postoperatively, VNRS scores for pain were lower in the pregabalin group (n=24) than in the placebo group (n=23; P<0.05). The number of patients who needed rescue analgesics was lower in the pregabalin group (P=0.042). The incidence of nausea and vomiting did not differ between groups (P=0.666), and the incidence of sedation was higher in the placebo groups (P=0.022).
Conclusion
The perioperative administration of oral pregabalin (150 mg twice) is an effective and safe way to reduce early postoperative pain in patients undergoing septoplasty.
Go to :

Keywords: Postoperative pain, Pregabalin, Septoplasty, Preemptive analgesia
INTRODUCTION
Septoplasty is widely performed to correct nasal septal deviation. To prevent postoperative bleeding and septal hematoma, nasal packing is usually applied. Unfortunately, nasal packing inevitably increases postoperative morbidity. Additional potential complications include worsening of sleep-disordered breathing, headache, and postoperative infection. Attempts have been made to eliminate the discomfort and pain caused by nasal packing [
1]. The procedures proposed are difficult to perform and some are impractical. A number of pain medications, such as opioids and nonsteroidal anti-inflammatory drugs (NSAIDs), are prescribed to decrease pain and to increase patient comfort during the nasal-packed state. Unfortunately, some of these painkillers also have potential side effects, including nausea, vomiting, urinary retention, sedation, and respiratory depression.
Dahl et al. [
2] described the use of anti-hyperalgesic drugs, such as gabapentin and pregabalin, as protective premedications prior to many different types of surgery. Protective medication is based on the principle that drugs given before an injury will reduce pain after the injury.
Pregabalin is a lipophilic analogue of gamma-aminobutyric acid and is being licensed for epilepsy, neuropathic pain, and generalized anxiety disorder. Its pharmacological profile is similar to gabapentin, but with higher potency and effectiveness. Although gabapentin and pregabalin were first identified as treatments for neuropathic pain and other neurologic disorders, several recent reviews have revealed that gabapentin and pregabalin also reduce postoperative opioid consumption and improve pain scores after spine surgery, laparoscopic surgery, and thyroidectomy [
3,
4,
5,
6,
7,
8]. To the best of our knowledge, no comprehensive data exist with regard to the analgesic efficacy of pregabalin in otolaryngologic patients undergoing septoplasty.
The aim of this randomized, double-blinded, placebo-controlled trial was to investigate the efficacy and safety of perioperative administration of pregabalin (150 mg twice a day) for one day in reducing acute postoperative pain in patients after septoplasty.
Go to :

MATERIALS AND METHODS
This study was approved by the Institutional Ethics Committee of Samsung Medical Center (IRB number: 2010-06-012) and is registered at
http://clinicaltrials.gov (registration number: NCT-01370915). Between October 2010 and January 2012, 47 patients with deviated septum who underwent septoplasty with or without turbinoplasty volunteered for this study. Written informed consent was obtained from all patients prior to randomization. Patients were excluded from the study if any of the following criteria were present: (1) age less than 20 years old or more than 60 years old; (2) known or suspected sensitivity or contraindication to pregabalin; (3) pregnant or breastfeeding; (4) use of chronic pain medications; (5) body mass index (BMI) ≥40 kg/m
2; (6) history of seizure disorder; (7) chronic renal insufficiency; or (8) motor or sensory disabilities.
Patients were randomly assigned to one of two groups to receive either pregabalin (Lyrica, Pfizer Inc., New York, USA) or placebo (vitamin complex). No other premedication was given to patients. Subjects in the experimental group received pregabalin (150 mg) one hour before surgery and then again 12 hours after the initial dose. In the control group, a vitamin complex served as placebo. A nurse who was blinded to the study administered all pills. All patients underwent conventional septolasty under general anesthesia. Nine patients underwent septoplasty with turbinoplasty using radiofrequency, and 2 patients using a debrider. After completing septoplasty, cotton pledgets with ointment were packed inside the nasal cavity to control bleeding. They were removed 2 days after operation. For acute pain relief, all patients received 650 mg acetaminophen 3 times a day after surgery.
The degree of pain and presence of side effects were examined every 6 hours for 48 hours. Pain was evaluated using an 11-point verbal numerical rating scale (VNRS), in which 0 represents no pain at all and 10 represents the worst pain imaginable. When a VNRS score was equal to or greater than 5 or a patient requested analgesics, additional injections of pethidine (50 µg IV) were allowed in the postanesthetic care unit or on the ward. Postoperative nausea and vomiting (PONV) were graded on a four-point scale, with 0, no nausea; 1, mild nausea; 2, severe nausea requiring antiemetics; and 3, retching and/or vomiting. Scores of 2 and 3 were grouped together to represent PONV, for which a rescue antiemetic (metoclopramide, 10 mg IV) was given. Sedation scores were recorded on a four-point scale, with 0, awake; 1, mild sedation; 2, sleepy, but arousable; and 3, very sleepy.
Statistical analysis was performed using SPSS ver. 14.0 (SPSS Inc., Chicago, IL, USA) and SAS 8.01 (SAS Institute, Cary, NC, USA). Patient characteristics (i.e., age, height, weight, and duration of surgery) were analyzed using the independent t-test. Categorical variables (rescue analgesics and side effects) were evaluated by Fisher exact test. Wilcoxon rank sum test with Bonferroni correction was used to compare VNRS scores for pain. Spearman partial correlation analysis with Bonferroni correction was used to correct for BMI and sex. All VNRS values were expressed as median±quartile range. P-values <0.05 were considered statistically significant.
Go to :

RESULTS
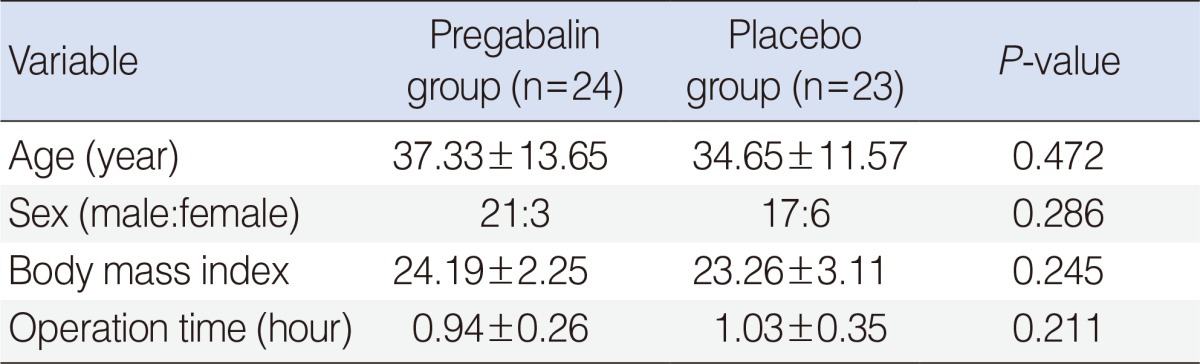
A total of 47 subjects received pregabalin or placebo after randomization, and all were included in data collection. Patient characteristics and surgery duration were similar for the two groups (
Table 1).
Table 1

VNRS scores and the presence of side effects were evaluated every 6 hours. Between 12 to 24 hours and 24 to 48 hours, VNRS scores were almost unchanged, so the highest score only during these periods was recorded. The presence of side effects was summed and graded as mentioned above, and also recorded as the highest score during same periods. Finally the degree of pain and side effects were grouped at 6, 12, 12 to 24, and 24 to 48 hours postoperatively. At 6 hours postoperatively, the mean VNRS score was 2.00 in the pregabalin group and 4.57 in the placebo group (
P=0.015). At 12 hours postoperative, the scores were 1.79 in the pregabalin group and 4.48 in the placebo group (
P=0.004). At 12 to 24 hours postoperative, scores were 2.75 in the pregabalin group and 4.74 in the placebo group (
P=0.072). At 24 to 48 hours postoperative, scores were 1.92 in the pregabalin group and 2.52 in the placebo group (
P=1.000). Multivariate analysis was also performed to correct for BMI and sex. Similar results to that of the prior analysis were obtained. Postoperative 6 and 12 hours VNRS results had a moderate inverse correlation with pregabalin medication administration (ρ=-0.4192 and -0.4692, respectively). The
P-values were 0.0185 and 0.0053, respectively (
Fig. 1).
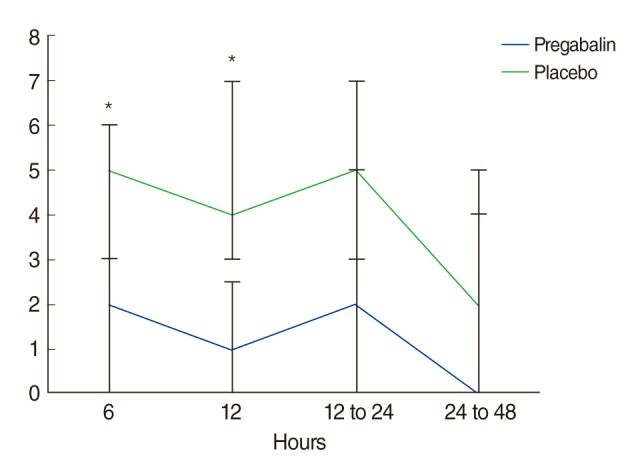 | Fig. 1Verbal numerical rating scale (VNRS) for pain at rest in placebo and pregabalin groups at 6, 12, 12 to 24, and 24 to 48 hours after surgery and after packing was removed. Spearman partial correlation analysis with Bonferroni correction. Values are median±quartile range. *P<0.05 compared with placebo. 
|
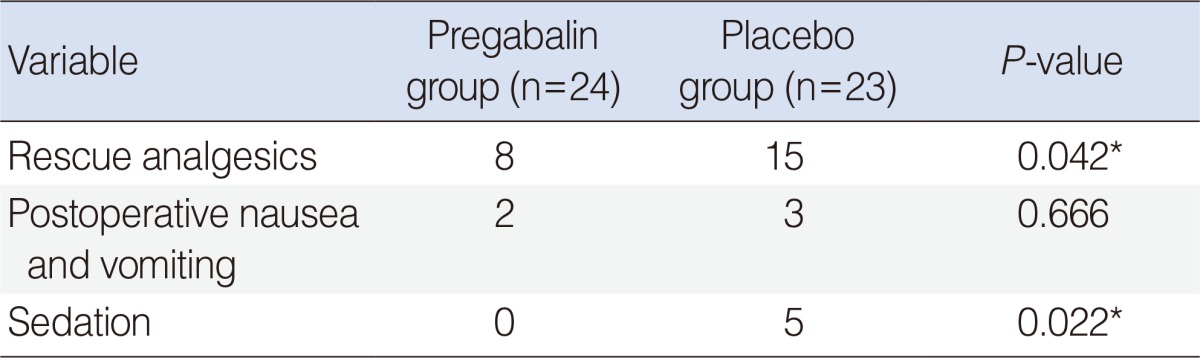
The number of rescue analgesics used during hospitalization was lower in the pregabalin group than in the placebo group (
P=0.042) (
Table 2). Incidences of PONV were similar in both groups (
P=0.666), and the incidence of sedation was higher in the placebo group (
P=0.022). Multivariate analysis on sedation scores could not be performed to correct for the use of additional analgesics because no patient complained about sedation in the pregabalin group. The incidence of PONV was low, and there were no sedation scores of 2 and 3 in either group. We analyzed the incidence of events as a sum of events with multivariate analysis, not the scores.
Table 2
Number of patients requiring rescue analgesics and the incidence of other side effects during the first 48 hours postoperatively

Go to :

DISCUSSION
Postoperative pain management is important for decreasing patient morbidity and pain-related clinical complications. Opioid and non-opioid analgesics are widely used to treat postoperative pain. However, opioids have significant side effects such as nausea, vomiting, sedation, and respiratory depression. Nasal packing is the leading cause of early postoperative pain, especially in septoplasty, but is deemed necessary to minimize postoperative complications such as hemorrhage, synechia formation, and septal hematoma. Some surgeons have used a suture technique without packing after septoplasty, but there is no consensus between these techniques [
1].
The transmission of pain signals evoked by tissue damage leads to sensitization of peripheral and central pain pathways. After activation of these pathways, a barrage of pain signals from the nociceptors leads to prolonged alterations in pain and touch sensation. Pain signals are amplified (hyperalgesia), and pressure signals are interpreted not as touch, but as pain (allodynia). Preemptive analgesia is a treatment initiated before a surgical procedure that reduces this sensitization thus decreasing pain severity and duration. Preemptive analgesia was first introduced by Woolf [
9] in 1983. Recently, an increasing number of published studies have focused on the preemptive analgesic use of gabapentin or pregabalin for postoperative pain relief [
3,
4,
5,
6,
7,
10,
11]. Although both gabapentin and pregabalin were first identified as treatments for neuropathic pain, pregabalin has been reported to be as effective for acute postoperative pain control [
3,
4,
5,
8,
11,
12,
13] as gabapentin [
2,
14,
15]. The pathogenesis of postoperative pain includes inflammatory, neurogenic, and visceral mechanisms. In these mechanisms Dahl et al. [
2] suggest that a transient or reversible type of neuropathic pain plays a major role in postoperative pain. This may explain why gabapentin and pregabalin, commonly used to treat chronic neuropathic pain, are effective for controlling postoperative pain.
In this study, we administered pregabalin orally at 1 hour before surgery and 12 hours after the initial dose with 650 mg acetaminophen 3 times a day after surgery. Given that the maximal pregabalin absorption time is about 1 hour and its half-life is about 6 hours [
16], we administered pregabalin 1 hour before surgery as a protective premedication. We also expected that a higher dose of pregabalin would contribute to a reduction in the development of chronic pain, so we repeated a 150-mg dose of pregabalin 12 hours after the initial dose [
6,
7]. Reuben et al. [
11] suggest that pregabalin can interact synergistically with COX-2 specific NSAIDs to reverse hyperalgesia associated with peripheral inflammation. The combination of pregabalin and NSAIDs was shown to be superior to either single drug alone for postoperative pain management. The mechanism of acetaminophen is weak COX-2 inhibitor and it is similar to COX-2 specific NSAIDs. Therefore the combination of pregabalin and acetaminophen also has synergistic effect on postoperative pain control.
The results of this randomized clinical trial suggest that the perioperative administration of oral pregabalin (150 mg twice, at one hour before surgery and 12 hours after the initial dose) is effective and safe in reducing early postoperative pain in patients undergoing septoplasty. Postoperative pain at 6 and 12 hours was significantly lower in the pregabalin group than in the control group. Between 12 to 24 and 24 to 48 hours, there was no significant difference in pain control. Preemptive pregabalin administration can reduce the need for additional analgesics in the postoperative period, but this difference is not statistically significant. The most common side effects of pregabalin are somnolence and dizziness, which occur more frequently at higher doses (400 to 600 mg per daily) [
17]. Kim et al. [
6] also showed that the incidence of side effects such as sedation and dizziness were higher in a group given a 150-mg dose of pregabalin than in a placebo group. In our study, however, PONV and sedation were not higher in the pregabalin group. We think that the control group reported PONV and somnolence more frequently because of rescue analgesics such as opioids. While they did occur, the sedation and PONV experienced by patients was tolerable without treatment. Without pregabalin, patients complained of more pain and needed additional rescue analgesics, which led to an increase in side effects such as PONV and sedation. Preemptive premedication with pregabalin may have a beneficial effect on patient management after septoplaty.
In conclusion, one day perioperative administration of oral pregabalin is an effective and safe way to reduce early postoperative pain in septoplasty patients.
Go to :






 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download