Abstract
Objectives
Obstructive sleep apnea syndrome (OSAS) is associated with repeated hypoxia and re-oxygenation. This characteristic of OSAS may cause oxidative stress and DNA damage. However, the link of OSAS with oxidative stress and DNA damage is still controversial. In the current study, we investigated whether OSAS causes DNA damage using alkaline single-cell gel electrophoresis (comet assay) and measuring oxidative stress by monitoring serum malondialdehyde (MDA) levels.
Methods
From March 2009 to August 2010, 51 patients who underwent polysomnography (PSG) during the night were enrolled in this study. We obtained serum from the patients at 6 AM. DNA damage and oxidative stress were evaluated using a comet assay and measuring serum MDA, respectively. We divided the patients into two groups according to the existence of comets appearing in the comet assay. Group 1 included 44 patients with negative assay results and group 2 consisted of seven patients with positive comet assay findings. We compared the age, gender proportion, PSG data (respiratory disturbance index [RDI], lowest O2 saturation level, and arousal index [AI]), time of disease onset, smoking habits, and serum MDA levels between the two groups.
Results
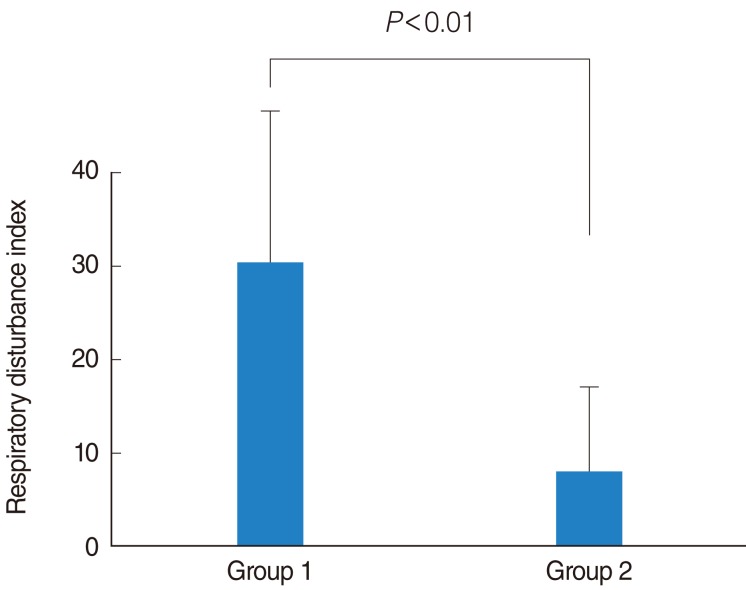
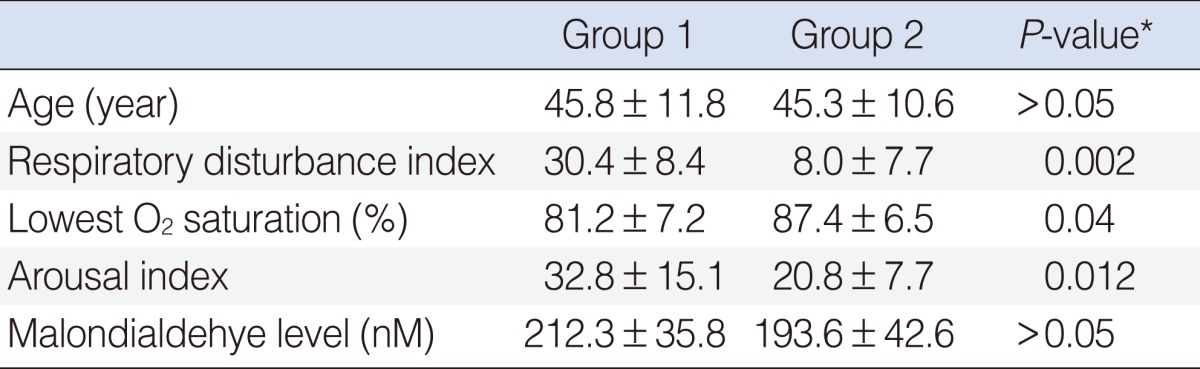
The average age and gender proportion of the two groups were not statistically different (P>0.05). The average of RDI for group 1 was 30.4±18.4 and 8.0±7.7 (P<0.01) for group 2. The average of lowest O2 saturation level for group 1 was 81.2±7.2 and 87.4±6.5 (P<0.05) for group 2. The average AI for group 1 was 32.8±15.1 and 20.8±7.7 (P<0.05) for group 2. Similarly, serum MDA levels of the two groups were not statistically different (P>0.05). No relationship between positive comet assay results and OSAS severity was identified.
Go to : 
Obstructive sleep apnea syndrome (OSAS) tends to cause episodic hypoxia-reoxygenation. Because of this, OSAS patients have been proposed as a human model for evaluating measurements of reactive oxidative species [1]. Oxidative stress and OSAS have pathophysiologic features that promote the development of atherosclerosis along with cardiovascular and neurodegenerative disease [2-4]. Many studies have reported that OSAS is associated with reactive oxidative stress (ROS) [5-7]. OSAS patients have increased levels of reactive oxygen metabolites in the blood that may result in cellular damage [8]. In addition, the level of DNA damage is higher in cases of OSAS than healthy individuals [9]. Oxidative stress and OSAS are also linked to endothelial dysfunction, which may predispose OSAS patients to cardio- and cerebrovascular diseases [10]. However, whether or not there are indeed increased levels of oxidative stress in OSAS patients is still uncertain [6,11,12]. Some studies have failed to support the hypothesis that oxidative stress is increased with OSAS [13,14]. These inconsistencies were due to factors associated with the OSA patients including the presence of co-morbidities and/or medications [15]. Plasma malondialdehyde (MDA) is a robust biomarker of oxidative stress; concentrations of this molecule are correlated with nocturnal desaturation levels below 85% [10]. One of the commonly used techniques for detecting DNA damage is single-cell gel electrophoresis (a comet assay). In the present study, we determined whether DNA damage and oxidative stress are associated with OSAS using a comet assay and measuring serum MDA levels.
Go to : 
This study was approved by the IRB of Gil Medical Center. Patients provided informed consent after receiving a complete explanation of our protocol. This was a prospective study. From March 2009 to August 2010, 51 patients who visited ENT department complaining snoring or obstructive sleep apnea were enrolled in this study. The exclusion criteria were patients with any type of cancer, previous chemotherapy or radiation therapy, bleomycin or antioxidant drugs, or underwent a computed tomography (CT) scan within the past 7 days. All patients underwent night polysomnography in Gil Medical Center. We obtained serum from the patients at 6 AM and performed a comet assay. We also measured serum MDA levels to evaluate oxidative stress. We analyzed the polysomnography data (respiratory disturbance index [RDI], lowest O2 saturation level, arousal index [AI], and time of disease onset), and compared the PSG and comet data from the two groups. In this study, the degree of OSAS was classified as mild (5≤RDI<15), moderate (15≤RDI<30), or severe (RDI>30) according to the RDI (apnea-hypopnea index+respiratory effort-related arousal).
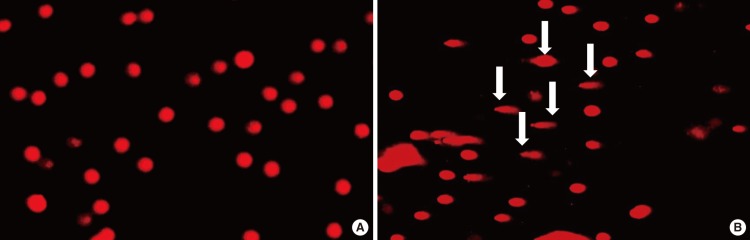
Alkaline single-cell gel electrophoresis (SCGE)/comet assay was performed as a three-layer procedure as previously described by Singh et al. [16] with slight modification. Briefly, blood was diluted in phosphate buffered saline (PBS, 1:1) and applied on top of a Ficoll (Lubon, Jiangsu, China) gradient (Sigma-Aldrich, St. Louis, MO, USA) and centrifuged at 400×g for 30 minutes. Peripheral blood monocytes were collected and washed twice with PBS. Separated monocytes were transferred into tubes, swelled with PBS, and centrifuged at 250×g for 10 minutes. Afterwards, the cells were mixed with 1% genetic technologic grade (GTG) agarose and applied to a 1% low electroendosmosis (LE) agarose pre-coated slide. Additional 1% GTG agarose was applied to the slide before it was immersed in lysis buffer (2.5 M NaCl, 100 mM EDTA, 10 mM Tris, 10% DMSO, and 1% Triton X-100) and incubated in the dark at 37℃ for 2 hours. The slide was washed in dextrose water and immersed in electrophoresis buffer (300 mM NaOH and 1 mM EDTA). Electrophoresis was performed (25 V, 300 mA) for 30 minutes in the dark at room temperature. After electrophoresis, the slide was washed three times with 0.4 M Tris for 10 minutes. After being fully dried, the slide was stained with ethidium bromide and examined under a fluorescence microscope (Nikon, Melville, NY, USA). We regarded a positive result from the comet assay when we could observe a comet appearance in at least one cell (Fig. 1B).
Free MDA was measured in plasma samples using high-performance liquid chromatography with ultraviolet ray (UV) detection after performing a derivatization step with 2-4-dinitrophenylhydrazine as previously described by Pilz et al. [17].
Differences in gender proportions and smoking habits were evaluated using Fisher's exact test. Differences in patient age, RDI, AI, lowest O2 saturation levels, and MDA levels between the groups were analyzed using a Mann-Whitney U-test with SPSS ver. 18.0 (SPSS Inc, Chicago, IL, USA). Differences between the groups were considered statistically significant when P<0.05.
Go to : 
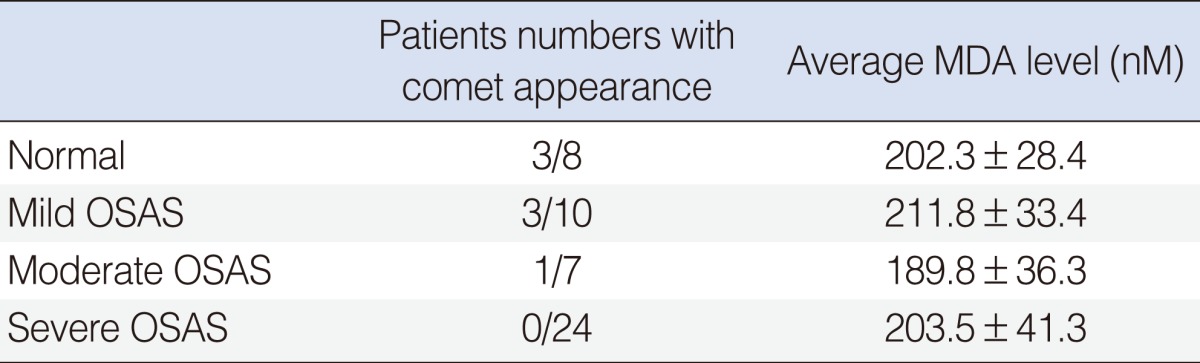
Among the 51 patients, there were 45 men and six women with a mean age of 45.7±11.5 years. Patients who did not have a comet appearing in the comet assay were enrolled in group 1 (n=44) and patients who had a comet appearing in comet assay were enrolled in group 2 (n=7) (Fig. 1). Group 1 included 40 men and four women while group 2 contained five men and two women. The gender proportions were not different statistically between the two groups (P>0.05). The mean of age of group 1 was 45.8±11.8 years and that of group 2 was 45.3±10.6 years (P>0.05). The average of RDI for group 1 was 30.4±18.4 while that of group 2 was 8.0±7.7. This difference was statistically different (P<0.01) (Fig. 2). Out of the 44 patients in group 1, 4 (9%) were normal, 20 (45%) had moderate OSAS, and 20 (45%) had severe OSAS. In group 2, 3 individuals (43%) were normal, 3 (43%) had mild OSAS, only 1 (14%) had moderate OSAS, and none had severe OSAS. According to OSAS severity, 3 of 8 normal patients showed comet appearance in their cells. Also, the same was true for 3 of 10 mild OSAS patients, 1 of 7 moderate OSAS patients and none of 24 severe OSAS patients. Normal and mild OSAS patients showed a higher incidence of comet appearance compared with moderate and severe OSAS patients (P<0.05) (Table 1). We have compared oxygen desaturation index (ODI) between the two groups. In group 1, the average ODI was 21.2±6.3, in group 2, it was 11.8±3.7, and the difference was statistically significant (P<0.05). The average lowest O2 saturation was 81.2%±7.2% in group 1 and 87.4%±6.5% in group 2 (P<0.05). The average of AI for group 1 was 32.8±15.1 and that for group 2 was 20.8±7.7 (P<0.05). The time during which these individuals had suffered from snoring or sleep apnea was also monitored and compared between the groups. In group 1, disease onset had occurred within 1 year in eight patients (18%), 1 to 5 years among 31 patients (70%), over 5 years ago among five individuals (11%). In contrast, disease onset occurred within 1 year in two patients (29%), 1 to 5 years in four patients (57%), and over 5 years ago in one individual (14%) in group 2. The time of disease onset was not statistically different between the two groups (P>0.05). There were 21 smokers (48%) in group 1 and 3 smokers (43%) in group 2. The number of smokers was not different statistically between groups (P>0.05). We were able to evaluate the levels of serum MDA. The average MDA level was 212.3±35.8 nM in group 1, and 193.6±42.6 nM in group 2 (P>0.05). According to OSAS severity, the average MDA level was 202.3±28.4 nM in normal patients, 211.8±33.4 nM in mild OSAS, 189.8±36.3 nM in moderate OSAS, and 203.5±41.3 nM in severe OSAS. Between the groups, the differences were not statistically significant (P>0.05) (Table 2).
Go to : 
The pathology of OSAS seems to involve repeated cycles of hypoxia during sleep. Cyclic changes in arterial oxygen saturation may increase the production of ROS. In the current study, we analyzed three markers of OSAS by PSG: RDI, the lowest O2 saturation index, and AI. Several biomarkers have been identified which can be used to measure oxidative damage. 8-OH-2-desoxyguanosine has been used to assess DNA damage caused by oxidative stress. Additionally, MDA can help evaluate the chemical modification of proteins prior to lipid oxidation. In vitro studies by Dyugovskaya et al. [6] showed increased expression of adhesion molecule and ROS production in leukocytes from OSAS patients. Schulz et al. [7] observed enhanced neutrophil superoxide release in OSAS patients compared to healthy controls. However, other studies have raised questions about the link between oxidative stress and OSAS. In vivo studies by Wali et al. [18] did not show differences in low-density lipoprotein susceptibility to oxidative stress when comparing OSAS patients and healthy controls. Ozturk et al. [13] evaluated glutathione, lipid peroxidation concentrations, and osmotic fragility in red blood cells, and failed to find increased oxidative stress in OSAS patients or differences in oxidative burst activity/capacity compared to healthy controls. In the current study, no correlation was found between DNA damage assessed by a comet assay and OSAS severity according to the RDI, AI, lowest O2 saturation values, and time of disease onset. Although we concluded that OSAS was not related to DNA damage or oxidative stress, other conditions such as hypertension and diabetes mellitus associated with sleep apnea may develop. Conflicting result from studies assessing oxidative stress levels may arise from methodological differences, difficulties with obtaining kinetic profiles or measuring ROS instability, and confounding factors such as malnutrition or renal dysfunction [4]. One study reported increased levels of oxidative stress at 9 PM OSAS patients with cardiovascular co-morbidities before going to sleep [11]. Another study found that plasma MDA levels in OSAS patients exhibit significant time-dependent variation with the lowest concentrations observed in the late evening, a progressive increase during the night, and the highest concentration occurring 1 hour after awakening [1]. In our present study, we showed that patients with moderate to severe OSAS did not display any evidence of increased DNA damage or oxidative stress than normal individuals or ones with mild cases of OSAS. We expected that the indices of oxidative stress and DNA damage would be increased in OSAS patients for several reasons. OSAS patients are subjected to disturbed hypoxic sleep. Thus, cyclic episodes of hypoxia/reoxygenation could facilitate free radical production that could result in oxidative stress and DNA damage. Wali et al. [18] did not find any significant differences in glutathione peroxidase or catalase activities in red blood cells from hypoxic and non-hypoxic patients. A lack of increased oxidative stress and DNA damage in OSAS patients could be explained by the hypothesis that OSAS does not initiate the generation of oxidative stress or promote DNA damage in the absence of significant comorbidities. This means that other factors which can affect oxidative stress and DNA damage have greater impacts than OSAS. This hypothesis is supported by the finding that continuous positive airway pressure therapy does not have any effect on antioxidant enzymes levels [15].
In summary, our data do not support the notion that OSAS promotes DNA damage or oxidative stress. Although obstructive sleep apnea is known to cause DNA and oxidative damages, the effect of OSAS is probably minimal. We therefore believe that other factors may play a greater role in promoting DNA damage and oxidative stress than OSAS. The causes of DNA damage and oxidative stress are multifactorial, and are likely affected by hypoxia, smoking, radiation, drug use, and nutrition along with a variety of additional risk factors. In our study, we found that OSAS is not a main factor for increased oxidative stress or DNA damage.
Go to : 
ACKNOWLEDGMENTS
This study was supported by a grant from the Bumsuk academic scholarship foundation.
Go to : 
References
1. Jordan W, Cohrs S, Degner D, Meier A, Rodenbeck A, Mayer G, et al. Evaluation of oxidative stress measurements in obstructive sleep apnea syndrome. J Neural Transm. 2006; 2. 113(2):239–254. PMID: 15959848.

2. Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993; 9. 90(17):7915–7922. PMID: 8367443.

3. Lavie L. Obstructive sleep apnoea syndrome: an oxidative stress disorder. Sleep Med Rev. 2003; 2. 7(1):35–51. PMID: 12586529.
4. Sofic E, Rustembegovic A, Kroyer G, Cao G. Serum antioxidant capacity in neurological, psychiatric, renal diseases and cardiomyopathy. J Neural Transm. 2002; 5. 109(5-6):711–719. PMID: 12111462.

5. Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ. 8-Isoprostane, a marker of oxidative stress, is increased in exhaled breath condensate of patients with obstructive sleep apnea after night and is reduced by continuous positive airway pressure therapy. Chest. 2003; 10. 124(4):1386–1392. PMID: 14555570.

6. Dyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med. 2002; 4. 165(7):934–939. PMID: 11934717.

7. Schulz R, Mahmoudi S, Hattar K, Sibelius U, Olschewski H, Mayer K, et al. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea: impact of continuous positive airway pressure therapy. Am J Respir Crit Care Med. 2000; 8. 162(2 Pt 1):566–570. PMID: 10934088.
8. Christou K, Markoulis N, Moulas AN, Pastaka C, Gourgoulianis KI. Reactive oxygen metabolites (ROMs) as an index of oxidative stress in obstructive sleep apnea patients. Sleep Breath. 2003; 9. 7(3):105–110. PMID: 14569521.

9. Kontogianni K, Messini-Nikolaki N, Christou K, Gourgoulianis K, Tsilimigaki S, Piperakis SM. DNA damage and repair capacity in lymphocytes from obstructive sleep apnea patients. Environ Mol Mutagen. 2007; 12. 48(9):722–727. PMID: 17973309.

10. Jordan W, Reinbacher A, Cohrs S, Grunewald RW, Mayer G, Ruther E, et al. Obstructive sleep apnea: Plasma endothelin-1 precursor but not endothelin-1 levels are elevated and decline with nasal continuous positive airway pressure. Peptides. 2005; 9. 26(9):1654–1660. PMID: 16112406.

11. Barcelo A, Miralles C, Barbe F, Vila M, Pons S, Agusti AG. Abnormal lipid peroxidation in patients with sleep apnoea. Eur Respir J. 2000; 10. 16(4):644–647. PMID: 11106206.

12. Lavie L, Vishnevsky A, Lavie P. Evidence for lipid peroxidation in obstructive sleep apnea. Sleep. 2004; 2. 27(1):123–128. PMID: 14998248.
13. Ozturk L, Mansour B, Yuksel M, Yalcin AS, Celikoglu F, Gokhan N. Lipid peroxidation and osmotic fragility of red blood cells in sleep-apnea patients. Clin Chim Acta. 2003; 6. 332(1-2):83–88. PMID: 12763284.
14. Muns G, Rubinstein I, Bergmann KC. Phagocytosis and oxidative burst of blood phagocytes in chronic obstructive airway disease. Scand J Infect Dis. 1995; 27(4):369–373. PMID: 8658072.
15. Svatikova A, Wolk R, Lerman LO, Juncos LA, Greene EL, McConnell JP, et al. Oxidative stress in obstructive sleep apnoea. Eur Heart J. 2005; 11. 26(22):2435–2439. PMID: 16105851.

16. Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988; 3. 175(1):184–191. PMID: 3345800.

17. Pilz J, Meineke I, Gleiter CH. Measurement of free and bound malondialdehyde in plasma by high-performance liquid chromatography as the 2,4-dinitrophenylhydrazine derivative. J Chromatogr B Biomed Sci Appl. 2000; 6. 742(2):315–325. PMID: 10901136.

18. Wali SO, Bahammam AS, Massaeli H, Pierce GN, Iliskovic N, Singal PK, et al. Susceptibility of LDL to oxidative stress in obstructive sleep apnea. Sleep. 1998; 5. 21(3):290–296. PMID: 9595608.
Go to : 




 Citation
Citation Print
Print


 XML Download
XML Download