This article has been
cited by other articles in ScienceCentral.
Abstract
Low grade nasopharyngeal papillary adenocarcinoma (LGNPPA) is an extremely rare variant of nasopharyngeal cancer, which exhibits distinct clinicopathological characteristics. Surgical resection has been regarded as the principal treatment. For this, transpalatal or transfacial approach has been classically used for exposure of the field. Up for now, there has been no report on applying endoscopic approach for this disease, which could be an effective alternative to minimize possible morbidities of palatotomy or maxillotomy. Endoscopic approach can be justified considering narrow extent and indolent behavior of LGNPPA. We report a patient with LGNPPA, which was successfully resected exclusively by endoscopic visualization. Our case exhibited narrow-based exophytic features with compatible immunopathologic profiles of LGNPPA. Exclusive endoscopic resection can be effective and less-morbid modality for this rare disease as in this case.
Go to :

Keywords: Nasopharyngeal neoplasms, Papillary adenocarcinoma, Endoscopy, Immunohistochemistry
INTRODUCTION
Low grade nasopharyngeal papillary adenocarcinoma (LGNPPA) is an extremely rare type of nasopharyngeal cancer. The clinicopathological characteristics of this disease were originally described from a series of nine consecutive cases [
1], and only five cases were reported thereafter [
2-
4]. Although the clinicopathological characteristics have not been completely determined due to the extreme rarity, the clinical characteristics is known to be distinct from those of the ordinary nasopharyngeal carcinomas [
1]. Surgical resection is regarded as the principal treatment [
2-
5]. Until now, resection has been performed employing potentially-morbid transpalatal or transfacial approach in all cases [
1-
3,
5]. Even though endoscopic nasal surgery has been adopted to the treatment of various nasal tumors, there has been no report for LGNPPA. Here we report a case of LGNPPA which was successfully treated with exclusive endoscopic resection via combined transnasal and transoral approach. This report was approved by the Institutional Review Board.
Go to :

CASE REPORT
An otherwise healthy 31-year-old female visited with complaints of nasal obstruction and mild postnasal drip for several weeks. Transnasal and transoral nasopharyngoscopic evaluations disclosed a tumor almost completely occluding nasopharynx (
Fig. 1A). The mass was exophytic, irregularly-surfaced, and very fragile even to endoscope-guided careful palpation and suction. Small tumor fragment was unintentionally dislodged during office-based endoscopic examination. Subsequent surface bleeding was only scanty without any problems, which suggested hypovascular feature of this tumor. Detailed examination of tumor attachment area and adjacent invasion was difficult in the office due to the excessive intracavitary bulging. Dislodged tumor dislodged piece of the tumor was sent for pathologic examination, which revealed the presence of LGNPPA. Computed tomography showed a 2.8×2.4 cm-sized, relatively hypovascular tumor without deep invasion or cervical lymphatic metastasis. The tumor exhibited typically exophytic feature. Through serial images, the point of stalk was estimated to be the narrow area around the cranial end of the nasal septum and the nasopharyngeal vault (
Fig. 1B). An 18-fluorodeoxyglucose positron emission tomography/computed tomography scan showed a mildly increased standardized uptake value of 2.55 without uptake elsewhere (
Fig. 1C). Thyroid ultrasonography revealed no thyroid nodule, especially no suspicious signs suggesting malignancy. Free T4, thyroid stimulating hormone, and thyroglobulin were all within the normal range.
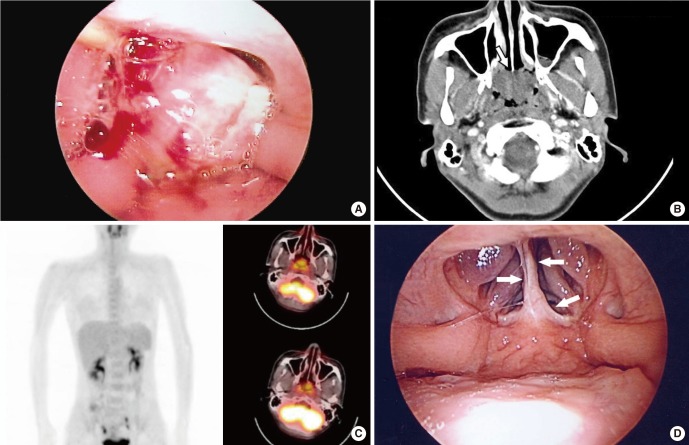 | Fig. 1A broad tumor occluding most of the nasopharyngeal cavity is shown with (A) a transoral nasopharyngoscopic view under soft palate retraction with a nelaton catheter under general anesthesia taken just before surgical exploration. The exophytic tumor occluded almost the entire nasopharyngeal cavity. (B) Computed tomography showing a 2.8×2.4 cm-sized pedunculated tumor without any submucosal infiltration. The location of the stalk was estimated to be confined around the nasopharyngeal vault and the posterior end of the nasal septum (arrow) without any adjacent invasion to parapharyngeal structures. (C) 18-FDG-positron emission tomography showing only mild 18-FDG uptake (standardized uptake value, 2.55) confined to the nasopharyngeal area. (D) Transoral nasopharyngoscopic finding 1 year after surgery. A focal fibrotic scar was observed in the area around the previously existing stalk (arrows). No recurrence was observed. FDG, fluorodeoxyglucose. 
|
Operative exploration under general anesthesia was planned both for evaluation and curative resection. Endoscopic exploration via combined transnasal and transoral approach was tried first, under preparation of further transpalatal or transfacial approach. At first, inferior and middle turbinates of both sides were lateralized or partially resected for visualization. The tumor, protruded into the choana of both nasal cavities, was carefully probed and mobilized. Area of tumor attachment was the narrow area in the nasal septum around choana and nasopharyngeal vault. Considering the tumor attachment confined only in narrow portion, we then decided to try exclusive endoscopic resection without transfacial or transpalatal approach. We tried to keep the resection margin to 0.5 cm around the tumor. Although en-bloc resection was originally planned, tumor was partially dislodged due to its fragility during the manipulation. But subsequent wider surgical field made the endoscopic resection more feasible. Mucosal incision was then made in the region around tumor stalk with sickle knife. Tissue was elevated from the underlying bony structure. This revealed no identifiable deep invasion and dissections were performed easily. Eventually, the mass and surrounding mucosa was successfully separated from the nasopharyngeal vault, and delivered transorally due to its large size.
The gross texture of the tumor was gritty, and fragile. The tumor was confined only to the shallow submucosal layer without deeper invasion. Although it was not encapsulated, resection margins were clear. It exhibited papillary growth pattern of glandular epithelial cells (
Fig. 2A). A thin fibrovascular core was visible without calcified psammoma bodies. The cells lining these structures had bland, round-to-oval, nuclei without mitotic figures (
Fig. 2B). Immunohistochemistry disclosed positivity for thyroid transcription factor-1 (TTF-1) (
Fig. 2C) and cytokeratin 7 (CK7), but negativity for thyroglobulin (
Fig. 2D) and CK20. These findings were considered identical to the previously described characteristics of LGNPPA [
1]. The patient was kept in hospital overnight and the posterior packing was removed the next day without any complications or morbidities. She did not receive adjuvant radiation therapy or chemotherapy. At 3 years after surgery, she remains free of disease (
Fig. 1D).
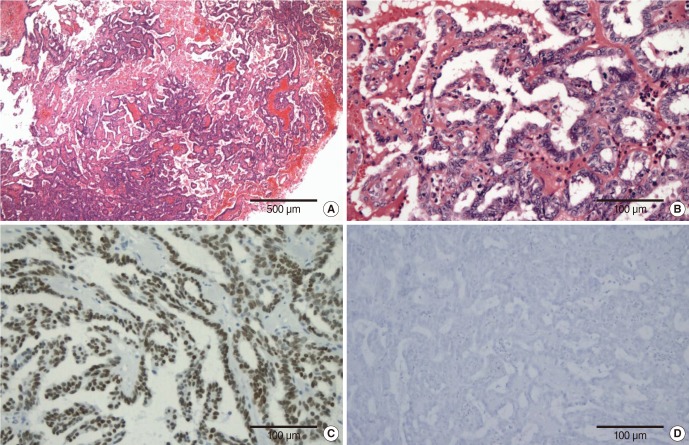 | Fig. 2(A) Low power view (×40) showing typical papillary growth pattern of glandular epithelial cells, which is usually seen in papillary thyroid carcinoma. (B) A thin fibrovascular core was visible without calcified psammoma bodies. The cells lining these structures had bland, round-to-oval, nuclei without mitotic figures (H&E, ×200). Immunohistochemistry disclosed (C) positivity for thyroid transcription factor-1 (×200) but (D) negativity for thyroglobulin (×200). 
|
Go to :

DISCUSSION
This case shows exclusive endoscopic resection via combined transoral and transnasal approach of LGNPPA is feasible and sufficient. Although only a few patients with LGNPPA have been described, their tumors were all considered 'radioresistant,' with definitive surgery being an effective and powerful therapy [
1-
3,
5]. There have been no reports of recurrence after surgical resection, whereas one patient failed radiation treatment, in which surgery was performed successfully as a salvage [
1].
Its biological behavior is uniquely insidious and indolent unlike most nasopharyngeal cancers. Hence, this is regarded as being between benign and malignant. However, the stromal invasion, the cytological pleomorphism, and recurrence following inadequate therapy have been regarded as indicatives of a malignant neoplasm, albeit a neoplasm of low grade [
1,
3].
To date, a transpalatal or transfacial maxillary swing approach to the nasopharynx has been used for all cases of LNGPPA [
1-
4]. However, these procedures can cause several complications, including pain, bleeding, and oronasal fistulae. Exclusive endoscopic resection can avoid these potential complications. In addition, LGNPPA is usually pedunculated, with limited extension extramucosally, which can make endoscopic resection feasible like this case.
The tumor in this case was attached to the nasopharyngeal vault and the posterior end of the nasal septum, which were effectively visualized exclusively by an endoscope. The narrowness of the stalk and minimal submucosal invasion made adequate resection margins possible. This case shows resection of LGNPPA with only endoscopic guidance can be sufficient for complete resection.
LGNPPAs arise from the surface epithelium. Unlike the majority of nasopharyngeal carcinomas, such as keratinizing or nonkeratinizing carcinomas, LGNPPA is thought to be a glandular neoplasm [
1]. More common glandular neoplasms are those originating from the submucosal seromucous salivary gland. Unlike this, LGNPPAs originate within surface mucosal glandular epithelia, a very rare pattern of pathogenesis among nasopharyngeal tumors. LGNPPA is a unique tumor type and much less common than nasopharyngeal salivary gland neoplasms [
1,
3].
Our case was positive for TTF-1 and CK7 but negative for thyroglobulin and CK20, a pattern identical to those previously reported [
1]. This pattern is similar to that of papillary thyroid carcinoma [
6], which is usually positive for TTF-1, CK7, and thyroglobulin, but negative for CK20. In addition, Histological characteristics of papillary growth of abundant glandular architecture are also similar in LGNPPA and thyroid papillary carcinoma [
1,
2]. Therefore, thyroid sonography has been recommended for LGNPPA patients, to identify any sign of hidden thyroid malignancies. This suggests that LGNPPA may be a form of metastatic disease originating from occult thyroid papillary carcinoma. To our knowledge, however, there have been no reports showing metastasis of papillary thyroid carcinoma into only the nasopharyngeal mucosa, with a confined pedunculated pattern and without any metastatic lymphadenopathy. Such a pattern was also observed in our patient, and we found no sonographic abnormality of thyroid. The clinicobiological similarity between LGNPPA and thyroid papillary carcinoma should be further investigated.
In conclusion, complete surgical resection has been deemed to be the treatment of choice for LGNPPA. This case was successfully treated with exclusive endoscopic resection via combined transnasal and transoral approach. Exclusive endoscopic resection was effective to obviate the potential morbidities of transpalatal or transfacial approach if performed appropriately in this rare variant of nasopharyngeal cancer.
Go to :







 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download