1. Guttman D, Stern Y, Shpitzer T, Ulanovski D, Druzd T, Feinmesser R. Expression of MMP-9, TIMP-1, CD-34 and factor-8 as prognostic markers for squamous cell carcinoma of the tongue. Oral Oncol. 2004; 9. 40(8):798–803. PMID:
15288834.

2. Pameijer FA, Balm A, Hilgers F, Muller SH. Variability of tumor volumes in T3-staged head and neck tumors. Head Neck. 1997; 1. 19(1):6–13. PMID:
9030938.

3. Johnson CR, Khandelwal SR, Schmidt-Ullrich RK, Ravalese J III, Wazer DE. The influence of quantitative tumour volume measurements on local control in advanced head and neck cancer using concomitant boost accelerated superfractionated irradiation. Int J Radiat Oncol Biol Phys. 1995; 6. 32(3):635–641. PMID:
7790249.
4. Brenner DE, Whitley NO, Houk TL, Aisner J, Wiernik P, Whitley J. Volume determinations in computed tomography. JAMA. 1982; 3. 247(9):1299–1302. PMID:
7062546.

5. Breiman RS, Beck JW, Korobkin M, Glenny R, Akwari OE, Heaston DK, et al. Volume determinations using computed tomography. AJR Am J Roentgenol. 1982; 2. 138(2):329–333. PMID:
6976739.

6. Lee RW, Mancuso AA, Saleh EM, Mendenhall WM, Parsons JT, Million RR. Can pretreatment computed tomography findings predict local control in T3 squamous cell carcinoma of the glottic larynx treated with radiotherapy alone? Int J Radiat Oncol Biol Phys. 1993; 3. 25(4):683–687. PMID:
8454487.

7. Freeman DE, Mancuso AA, Parsons JT, Mendenhall WM, Million RR. Irradiation alone for supraglottic larynx carcinoma: can CT findings predict treatment results? Int J Radiat Oncol Biol Phys. 1990; 8. 19(2):485–490. PMID:
2394626.

8. Gilbert RW, Birt D, Shulman H, Freeman J, Jenkin D, MacKenzie R, et al. Correlation of tumour volume with local control in laryngeal carcinoma treated by radiation therapy. Ann Otol Rhinol Laryngol. 1987; Sep-Oct. 96(5):514–518. PMID:
3674647.
9. Mukherji SK, Mancuso AA, Mendenhall W, Kotzur IM, Kubilis P. Can pretreatment CT predict local control of T2 glottic carcinomas treated with radiation therapy alone? AJNR Am J Neuroradiol. 1995; 4. 16(4):655–662. PMID:
7611018.
10. Spiro RH, Huvos AG, Wong GY, Spiro JD, Gnecco CA, Strong EW. Predictive value of tumor thickness in squamous carcinoma confined to the tongue and floor of the mouth. Am J Surg. 1986; 10. 152(4):345–350. PMID:
3766861.

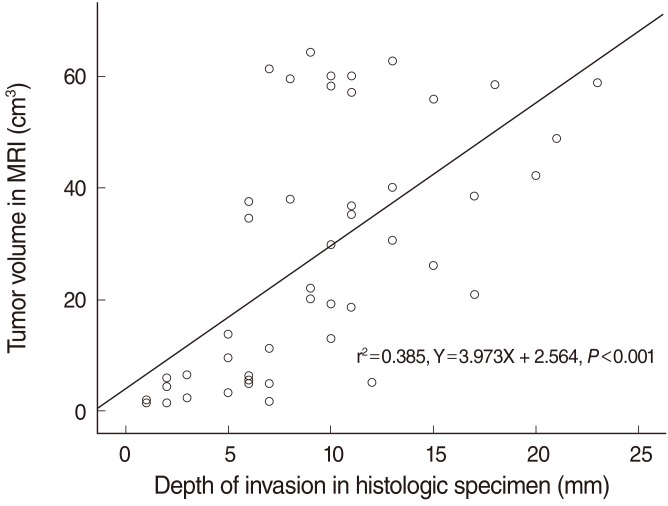
11. Kim SH, Cho NH, Kim K, Lee JS, Koo BS, Kim JH, et al. Correlations of oral tongue cancer invasion with matrix metalloproteinases (MMPs) and vascular endothelial growth factor (VEGF) expression. J Surg Oncol. 2006; 3. 93(4):330–337. PMID:
16496371.

12. Iwai H, Kyomoto R, Ha-Kawa SK, Lee S, Yamashita T. Magnetic resonance determination of tumour thickness as predictive factor of cervical metastasis in oral tongue carcinoma. Laryngoscope. 2002; 3. 112(3):457–461. PMID:
12148854.
13. Brenner DJ. Dose, volume and tumor control predictions in radiotherapy. Int J Radiat Oncol Biol Phys. 1993; 4. 26(1):171–179. PMID:
8482624.
14. Johnson CR, Thames HD, Huang DT, Schmidt-Ullrich RK. The tumor volume and clonogen number relationship: Tumor control predictions based upon tumor volume estimates derived from computed tomography. Int J Radiat Oncol Biol Phys. 1995; 9. 33(2):281–287. PMID:
7673015.
15. Chua DT, Sham JS, Kwong DL, Tai KS, Wu PM, Lo M, et al. Volumetric analysis of tumor extent in nasopharyngeal carcinoma and correlation with treatment outcome. Int J Radiat Oncol Biol Phys. 1997; 10. 39(3):711–719. PMID:
9336154.

16. Willner J, Baier K, Pfreundner L, Flentje M. Tumor volume and local control in primary radiotherapy of nasopharyngeal carcinoma. Acta Oncol. 1999; 38(8):1025–1030. PMID:
10665757.

17. Johnson CR, Khandelwal SR, Schmidt-Ullrich RK, Ravalese J 3rd, Wazer DE. The influence of quantitative tumor volume measurements on local control in advanced head and neck cancer using concomitant boost accelerated superfractionated irradiation. Int J Radiat Oncol Biol Phys. 1995; 6. 32(3):635–641. PMID:
7790249.

18. Gilbert RW, Birt D, Shulman H, Freeman J, Jenkin D, MacKenzie R, et al. Correlation of tumor volume with local control in laryngeal carcinoma. Ann Otol Rhinol Laryngol. 1987; Sep-Oct. 96(5):514–518. PMID:
3674647.
19. Kuriakose MA, Loree TR, Hicks WL, Welch JJ, Wang H, DeLacure MD. Tumour volume estimated by computed tomography as a predictive factor in carcinoma of the tongue. Br J Oral Maxillofac Surg. 2000; 10. 38(5):460–465. PMID:
11010774.

20. Chew MH, Khoo JB, Chong VF, Tai BC, Soo KC, Lim DT. Significance of tumour volume measurements in tongue cancer: a novel role in staging. ANZ J Surg. 2007; 8. 77(8):632–637. PMID:
17635274.

21. Been MJ, Watkins J, Manz RM, Gentry LR, Leverson GE, Harari PM, et al. Tu mor volume as a prognostic factor in oropharyngeal squamous cell carcinoma treated with primary radiotherapy. Laryngoscope. 2008; 8. 118(8):1377–1382. PMID:
18418275.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download