MATERIALS AND METHODS
The study was assessed in the ENT Department of Kirikkale University Faculty of Medicine. The immunohistochemical staining and light microscopic examination was performed by Pathology Department of Kirikkale University Faculty of Medicine.
Subjects
The NP group was selected from the patients examined by the ENT Department of Kirikkale University Faculty of Medicine. They have used topical corticosteroid nasal spray for a duration of 6-week and if the pathology was ongoing, the operation was performed. This study group consisted of 24 adult patients (21 males, 3 females) with nasal polyp who underwent functional endoscopic sinus surgery (FESS) under general anesthesia. Patients ranged from 23 to 70 years; and the mean age was 46.5±11.2 years. All patients in the study group answered a questionnaire, and underwent ENT examination, endoscopic examination with 0° and 30° endoscopes, Water's view, and axial and coronal computed tomography (CT) of paranasal sinuses. During FESS, specimens including polyp tissues were excised from 3 regions: nasal cavity, maxillary, and ethmoid (anterior and posterior) sinuses. They were only examined at ×400 magnification under light microscopy and the slides with polypoid tissues were also included in the study. The tissues, which were edematous and rich in vessels, had severe inflammatory cells and showed polypoid developments, and were thus included into the study as the polyp group. Slides including chronic inflammatory process without polypoid tissue were excluded from the study. Finally, the study group consisted of three regions: ethmoid sinuses (including 14 specimens), maxillary sinus (including 10 specimens), and nasal cavity (including 10 specimens).
The control group consisted of 9 adult patients without NP (6 males and 3 females) who underwent septoplasty operation. Their ages ranged between 18 and 55 years; and the mean age was 28.22±12.24 years. In this group, specimens were collected via punch biopsies from inferior turbinates that were normal and non-hypertrophied during septoplasty, and 9 specimens were included into the control group. The subjects in the control group were accepted to enter the study with written approvals.
In the NP groups, the polyp duration was assessed by hospital data and patient history including the first diagnosis time of NP and follow-ups of data. There were no other diseases in subjects of both groups.
Method
Questionnaire
In the questionnaire form, the anterior and posterior nasal discharge, nasal congestion, cough, facial and dental pain, halitosis, paroxysmal nocturnal coughing spells, sore throat, fever, olfactory loss, headache, and ear pain were all asked [
6]. Smoking status (yes or no) and Brinkman Index (daily number of cigarettes×years of smoking) were also noted [
7].
Endoscopic examination
Endoscopic examination with 0° and 30° endoscopes were performed in Endoscopy Unit of ENT Department of Kirikkale University Faculty of Medicine. Discharge (none, clear and thin, thick, purulent), mucosal status (normoplasia, light hyperplasia with no erythema, hyperplasia) [
8], anatomic anomalies (septal deviation, lateral rotation of the uncinate process, turbinate hypertrophy, and other anatomic anomalies) [
6], and localizations and sizes of the polyps were examined.
In the preoperative nasal endoscopic examination of study group, the appearance of NPs was staged as Lawson's (1991) [
9]. Stage 0, no polyp presented; stage 1, polyp under medial turbinate that was seen by endoscopy; stage 2, protruding polyp in medial turbinate that was seen without using endoscopy; stage 3: massive polyposis.
Computed tomography
From the axial and coronal sections of paranasal sinuses in NP group, the localizations and sizes of the polyps in the nasal cavity and paranasal sinuses were evaluated. And also, the pan-polyposis, septal deviation, concha bullosa, lateral rotation of the uncinate process, prominent ethmoid bulla, and other anatomic abnormalities [
6] were also investigated. In the control group, both coronal and axial computed tomographic evaluations were assessed.
Immunohistochemical staining
In the study and control groups, surgical specimen was examined with immunohistochemical staining techniques with monoclonal antibodies against PDGF. In each of the surgical specimens, the number of PDGF positivity were evaluated in 3-4 high magnification fields on light microscope; and, the mean number of these cells in the EP, subepithelial layer of lamina propria (SE) and deep paraglandular layer of the mucosa (D) were determined.
Immunohistochemical staining technique
Five-micron-thick sections were obtained, transferred on to adhesive slides, and dried in autoclave at 37℃ overnight and at 60℃ for 20 minutes. They were deparaffinized and dehydrated by immersion into xylene twice for ten minutes and in alcohol twice for ten minutes. The specimen was then incubated in 3% H2O2 for five minutes to inhibit endogenous peroxidases. The preparations were transferred into citrate-based antigen retrieval solutions (Dako, Glostrup, Denmark; pH:6) for PDGF antigens (Lab Vision Co., Neomarkers, Fremont, CA, USA). All slides were kept in microwave oven (750 watts) twice for five minutes. The Sequeza Tm manual staining device (Shandon Scientific, Astmoor, UK) for standardization was used, the classical streptavidin avidin-biotin-peroxidase methods and the diaminobenzidine chromogen (20 minutes) were applied for immunohistochemical analysis of three antibodies. Non-immune mouse serum was served as a negative control and Mayer's haematoxylin was used as counterstain. Cytoplasmic staining was considered as evidence of positivity.
The slides were reviewed by an expert pathologist. In each slide, in order to evaluate the inflammatory cells, the initial hematoxylin and eosin (H&E) sections were prepared and examined by light microscope. On H&E sections, we observed inflammatory cells (polymorpho nuclear cells [PMNCs], including polymorpho nuclear leukocytes and eosinophils; mononuclear cells [MNCs] including lymphocytes, plasma cells, mast cells and histiocytes; and in all cells group, there were additionalfibroblasts of PMNCs and MNCs). After explaining the first stage above, at the second stage, the PDGF expressions and the number of inflammatory cells were counted by light microscope (Leica, Germany) per 100 cells in 3-4 high magnification fields. Means of cell counts were calculated as percentage (%) values; and the PDGF PI values at inflammatory cells were also detected on 0-3 scale.
Positivity index
For the quantitative assessments of the PDGF expression, staining in the EP, SE, and deep layers of the lamina propria, and the inflammatory cells were assessed by counting totally 100 cells in 3-4 high magnification field and the means were calculated. Eventually, the means of the PDGF (+) cells as per 100 cells on a high magnification field (×400) were detected in the E, SE and deep layers of the lamina propria. Scoring was performed on a 0-3 scale, where 0 represented negative staining: 1) weakly positive; 2) positive; and 3) strongly positive [
10]. PI 0 means that antigen (PDGF)+ cell count was 0% (no stained cells); PI 1 means that antigen (PDGF)+ cell count was <5%; PI 2 means that antigen (PDGF)+ cell count was 5%-50%; PI 3 means that antigen (PDGF)+ cell count was >50%.
Assessment was performed at eight levels: 1) epithelial_apical (EP_apical), 2) epithelial_basal (EP_basal), 3) subepithelial_perivascular (SE_pv), 4) subepithelial_glandular (SE_gland), 5) subepithelial_endothelial (SE_endothelial), 6) deep_perivascular (D_pv), 7) deep_glandular (D_gland), 8) deep_endothelial (D_endothelial).
Statistical analysis
Statistical packet for SPSS ver. 16.0 (SPSS Inc., Chicigo, IL, USA) was used for statistical evaluations. For all four groups; namely the nasal cavity, maxillary sinus, ethmoid sinus and control groups, the differences for the PDGF PI between groups were analyzed by Kruskal-Wallis variance analysis. When a statistically significant result was found, the pairwise comparisons were done by "Mann Whitney U-test" with Bonferroni corrections to detect the value of group, which had caused difference.
In the study group, correlations between age, gender, polyp duration, smoking and Brinkman Index values, and the PDGF levels in ethmoid sinus, maxillary sinus and nasal cavity were analyzed separately by Spearman's correlation Rho efficient for groups 1-3 separately.
The confounding factors (covariates age, gender, polyp duration, smoking and Brinkman Index, PMNC-%, MNC-%, PDGF-PMNC, PDGF-MNC, PDGF-all cells) affecting PDGF-PI in layers of mucosa (epithelial, subepithelial, and deep) were analyzed by linear regression analysis (backward, step by step analysis) in the polyp groups (groups 1-3) totally. A P-value <0.05 was considered statistically significant.
All steps of the study were planned and conducted with approval of Kirikkale University Faculty of Medicine Local Ethique Committee (date, March 23th 2009; number, 2009/028), and confirmed to the principles outlined in the Declaration of Helsinki [
11]. This study was supported by "Kırıkkale University Scientific Research Projects Unit Funds".
Go to :

RESULT
Questionnare results showed that patients with NP in groups 1-3 were disturbed from nasal discharges and obstructions, and their CT findings revealed polyposis in maxillary and/or ethmoid sinuses with no additional anatomic abnormalies being observed.
PDGF PI values in EP_apical, EP_basal, SE_pv, SE_gland, SE_endothelial, D_pv, D_gland, and D_endothelial, parts of ethmoid sinus, maxillary sinus, nasal cavity and control groups, together with MNC-% and PGDF-PI, PMNC-% and PGDF-PI and all cells PGDF-PI were demonstrated as median on
Table 1 and
Figs. 1-
3.
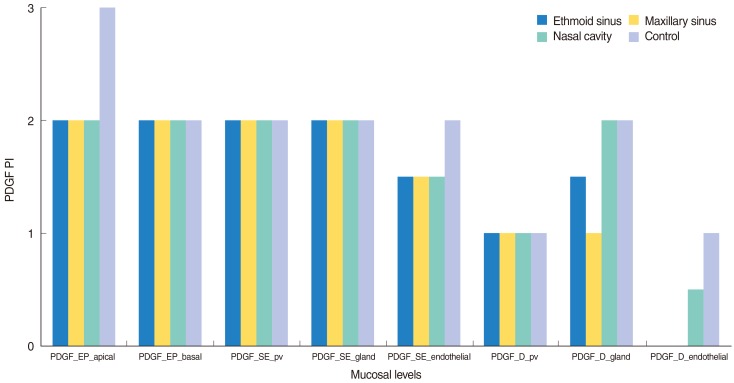 | Fig. 1Platelet-derived growth factor (PDGF) positivity index (PI) levels in mucosa of groups 1-4. EP, epithelium; SE, subepithelial layer of lamina propria; pv, perivascular; D, deep paraglandular layer of the mucosa. 
|
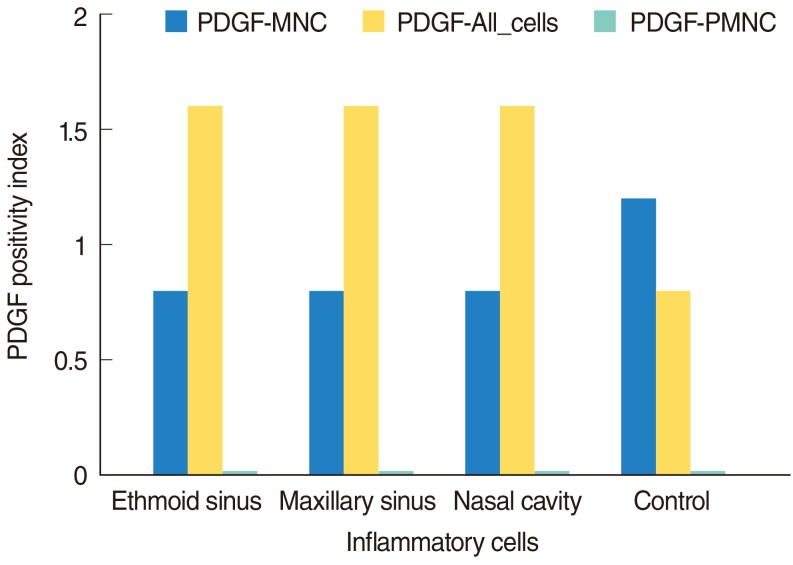 | Fig. 3Platelet-derived growth factor (PDGF) positivity indexs in inflammatory cells. MNC, mononuclear cell, PMNC, polymorphonuclear cell. 
|
Table 1
PDGF PI levels in ethmoid sinus, maxillary sinus, nasal cavity, and control groups
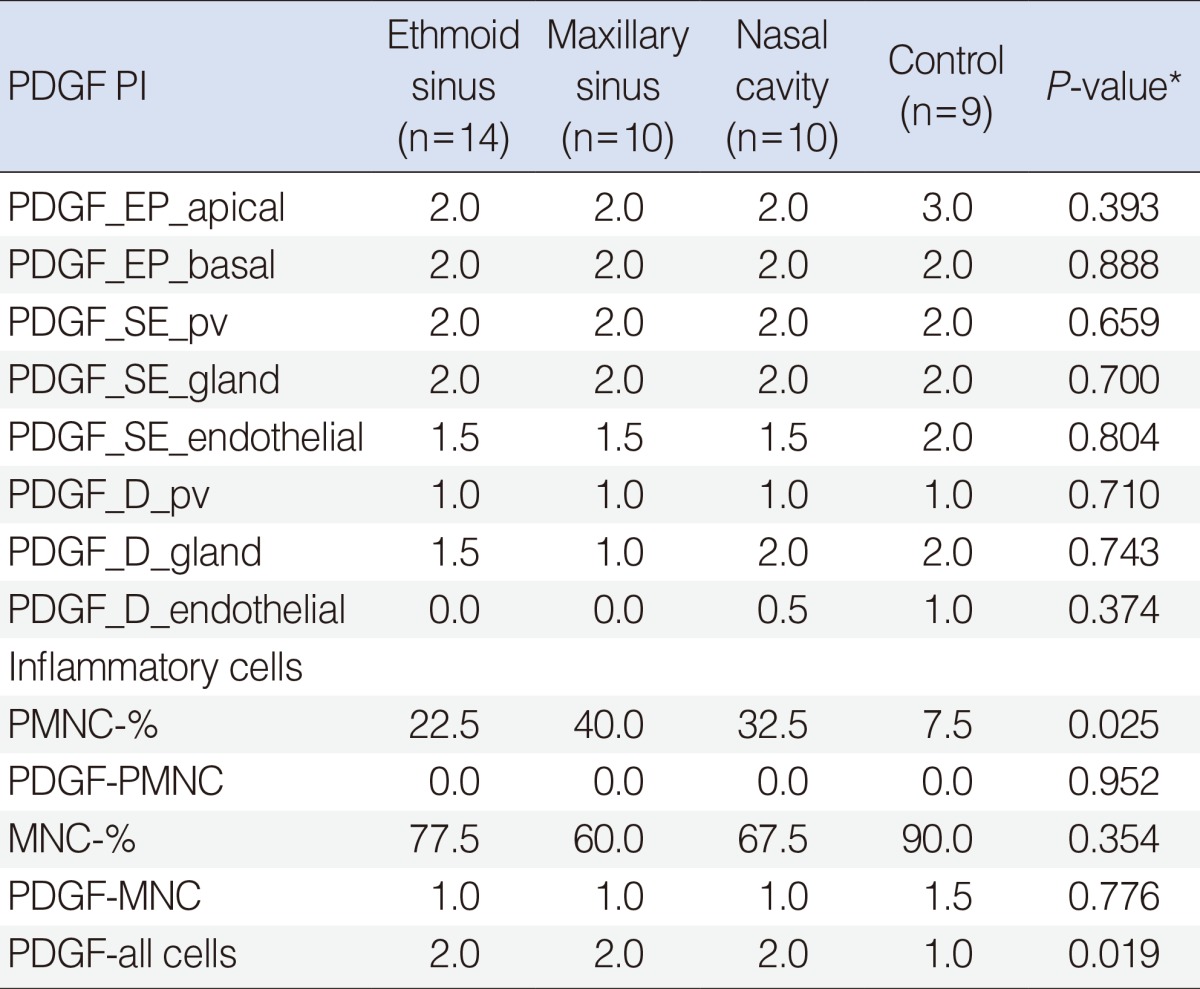

The difference for PDGF PI values at eight layers of mucosa between all four groups was analyzed by Kruskal-Wallis variance analysis. No statistically significant differences were found between groups (
Table 1). The differences between inflammatory cells-% (PMNC-%, MNC-%); and PDGF PI of PMNC with the MNC and all cells were analyzed by Kruskal Wallis variance analysis; the statistically significant difference was found for PMNC-% (
P=0.025) and PDGF-all_cells (
P=0.019) between groups 1-4 (
Table 1).
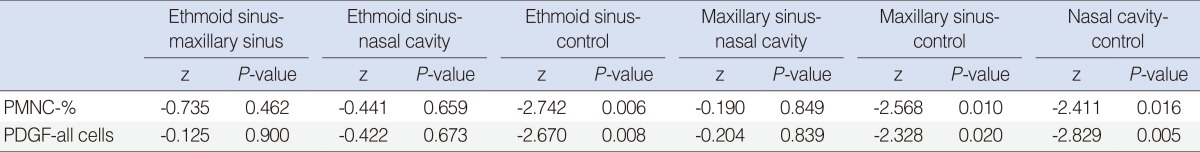
To find the value which caused the difference, pairwise comparisons were performed by Mann Whitney
U-test with Bonferroni correction. PMNC-% values of ethmoid sinus (median, 22.5;
P=0.006); and maxillary sinus (median, 40.0;
P=0.010) were significantly higher than that of the control group (median, 7.5). The PDGF PI of all cells of ethmoid sinus (median, 2.0;
P=0.008) and nasal cavity (median, 2.0;
P=0.006) were significantly higher than that of the control group (median, 1.0) (
Table 2).
Table 2
Pairwise comparisons by Mann Whitney U-test with bonferroni correction*

In the study group, correlations between age, gender, polyp duration, smoking and Brinkman Index values together with the PDGF levels in ethmoid sinus, maxillary sinus, and nasal cavity were analyzed separately by Spearman's correlation Rho efficient. In older patients, PDGF_D_gland PI values decreased (P=0.014, r=-0.640) in ethmoid sinus. In nasal cavity polyps, as the duration extended, PDGF_D_endothelial positivity values were also increased (P=0.004, r=0.913).
The confounding factors (covariates age, gender, polyp duration, smoking and Brinkman Index, PMNC-%, MNC-%, PDGF-PMNC, PDGF-MNC, and PDGF-all cells) affecting PDGF-PI in layers of mucosa (epithelial, subepithelial and deep) were analyzed by linear regression analysis (backward, step by step analysis)(
Table 3):
Table 3
Linear regression (backward) analysis of factors affecting PDGF-PI levels at eight layers of mucosa in polyp groups (groups 1-3)
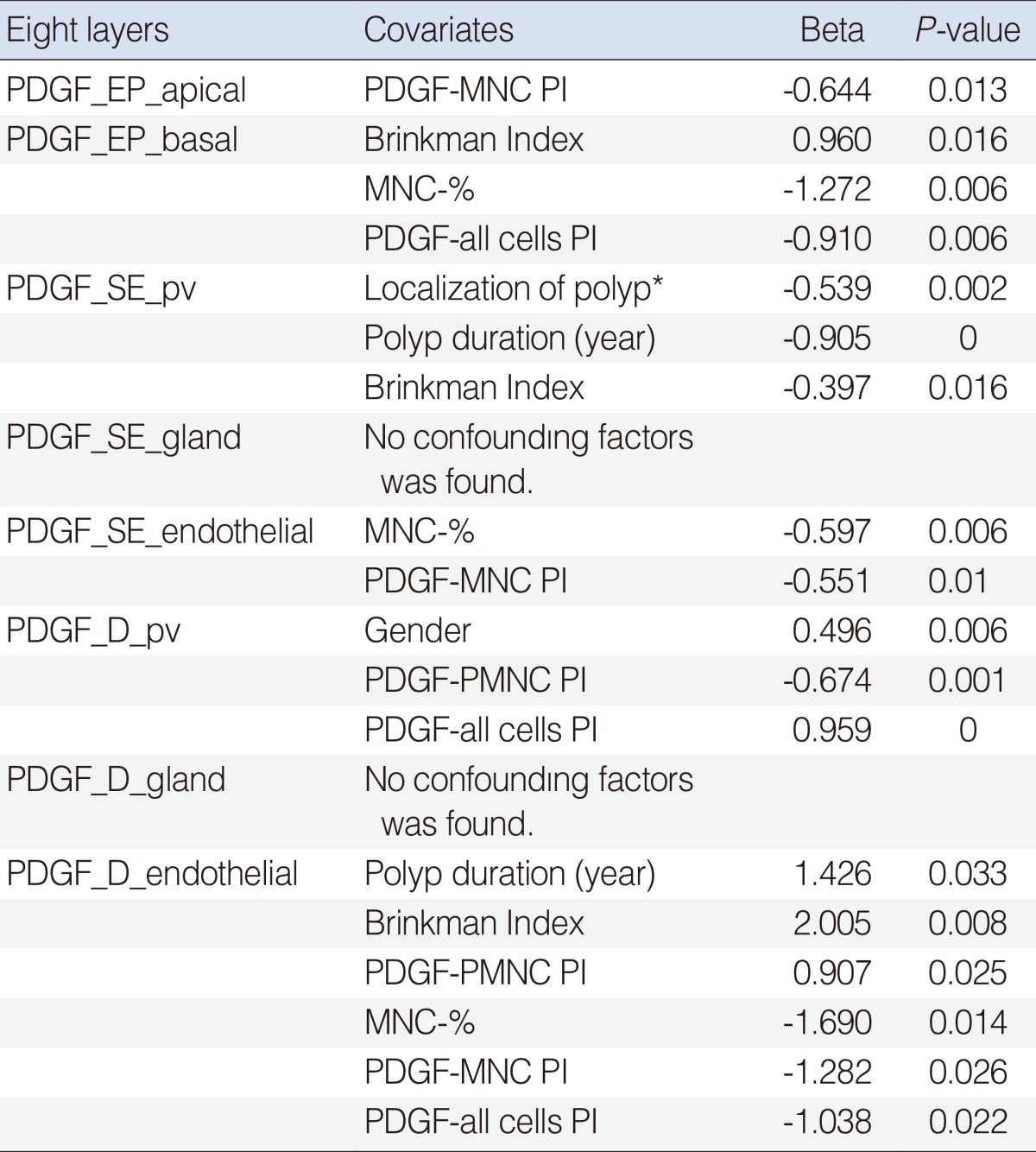

For PDGF_EP_apical PI
1) As PDGF-MNC PI increased, PDGF_EP_apical PI decreased (P=0.013, Beta=-0.644).
For PDGF_EP_basal PI
1) As Brinkman Index increased, PDGF_EP_basal PI increased (P=0.016, Beta=0.960). 2) As MNC-% (P=0.006, Beta=-1.272) and PDGF-all cells PI (P=0.006, Beta=-0.910) increased, PDGF_EP_basal PI decreased.
For PDGF_SE_pv PI
1) In ethmoid and maxillary sinuses, PDGF_SE_pv PI values increased; and in nasal cavity polyps, PDGF_SE_pv PI values decreased (P=0.002, Beta=-0.539). 2) In older polyps, PDGF_SE_pv PI values decreased (P=0.000, Beta=-0.905). 3) As Brinkman Index values increased, PDGF_SE_pv PI values decreased (P=0.016, Beta=-0.397).
For PDGF_SE_gland PI
No significant confounding factors was found (P>0.05).
PDGF_SE_endothelial PI
As MNC-% (P=0.006, Beta=-0.597); and PDGF-MNC PI (P=0.010, Beta=-0.551) increased, PDGF_SE_endothelial PI decreased.
For PDGF_D_pv PI
1) In male polyp patients, PDGF_D_pv PI increased (P=0.006, Beta=0.496). 2) As PDGF-PMNC PI increased, PDGF_D_pv PI decreased (P=0.001, Beta=-0.674). 3) PDGF-all_cells PI increased, PDGF_D_pv PI increased (P=0.000, Beta=0.959).
For PDGF_D_gland PI
No significant confounding factors was found (P>0.05).
PDGF_D_endothelial PI
1) In older polyps, PDGF_D_endothelial PI increased (P=0.033, Beta=1.426). 2) As Brinkman Index increased, PDGF_D_endothelial PI increased (P=0.008, Beta=2.005). 3) As PDGF-PMNC PI increased, PDGF_D_endothelial PI increased (P=0.025, Beta=0.907). 4) As MNC-% increased, PDGF_D_endothelial PI decreased (P=0.014, Beta=-1.690). 5) As PDGF-MNC PI increased, PDGF_D_endothelial PI decreased (P=0.026, Beta=-1.282). 6) As PDGF-all cells PI increased, PDGF_D_endothelial PI decreased (P=0.022, Beta=-1.038).
Histopathologic findings
On light microscopic examinations, it was observed that pseudostratified ciliated EP was present in majority of the polyps; and a very small part of polyps was also lined with metaplastic EP. In PDGF stained sections, diffuse positivity was detected in both the surface and the mucosal gland EP. Since the inflammatory infiltrates and fibroblastic activities are more predominant in the subepithelial and perivascular locations, the PDGF positivity was higher around the glands and blood vessels of subepithelial and deep layers of lamina propria. Vascular endothelial cells, especially the ones located superficially, also showed positive staining with PDGF. In edematous areas of polyps, the expression of all markers was found to be less than those in other areas. This was concluded to be due to the small number of cells in these edematous areas (
Figs. 4-
6).
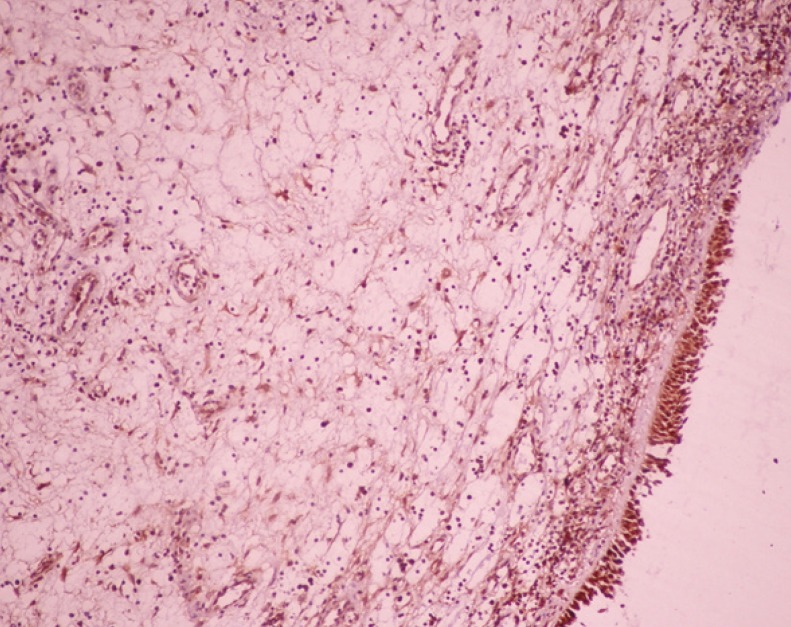 | Fig. 4Immunohistochemical staining for platelet-derived growth factor (PDGF) in polyp sample displaying decreases from epithelial apical to deep layers of lamina propria. Positive staining for PDGF was mainly observed in cytoplasms of mononuclear cells at epithelial apical and subepithelial layers; and fibroblasts at deep layers (×100). 
|
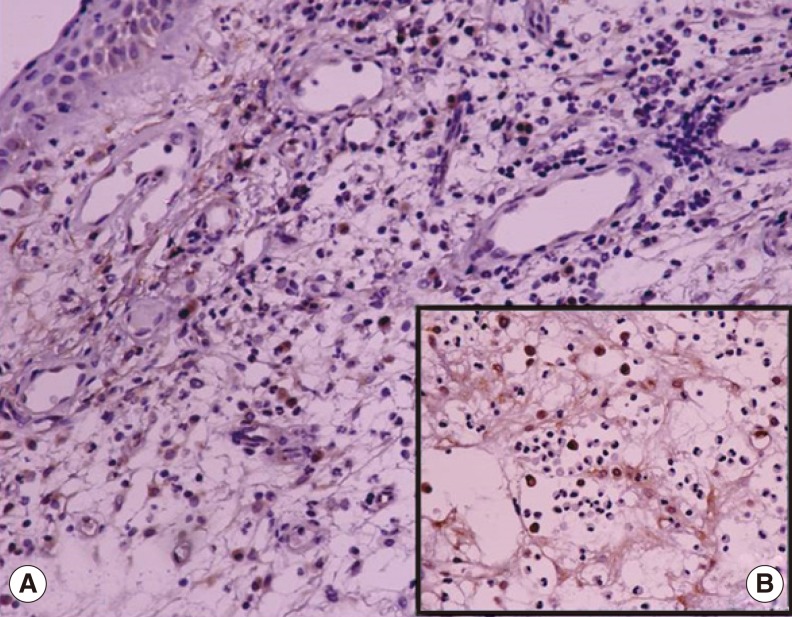 | Fig. 6Immunohistochemical staining for platelet-derived growth factor (PDGF) in polyp sample shows that: (A) No-staining was observed in polymorphonuclear cells (PMNCs); and positive staining was observed in mononuclear cells and fibroblasts at subepithelial layer of lamina propria (×200). (B) The +3 PDGF positivity was detected in cytoplasms of plasma cells; and +2 PDGF positivity was detected in cytoplasms of fibroblasts. No positive staining for PDGF was observed in cytoplasms of PMNCs (×400). 
|
Go to :

DISCUSSION
NPs generally originate from the ethmoid cells and consist of an epithelial lining surrounding underlying edema, glandular hyperplasia, fibrosis and eosinophilic infiltration. The polypoid stroma is highly edematous with a varying density of inflammatory cells. The pathophysiology of NPs' remain poorly understood [
12].
PDGF is one of the numerous growth factors, namely the vascular endothelial growth factors B and C [
13,
14] and placental growth factor, that regulates cell growth and division. In particular, it plays significant roles in blood vessel formation (angiogenesis), the growth of blood vessels from already-existing blood vessel tissues [
15]. The PDGF is a potent mitogen for cells of mesenchymal origin, including smooth muscle cells and glial cells [
16,
17]; and, it plays a role in embryonic development, cell proliferation, cell migration, and angiogenesis [
18].
Tissue remodelling in NP as well as in bronchial tissues includes sub-basement membrane depositions of collagen, stromal deposition of extracellular matrix protein, and hypertrophy/hyperplasia of airway smooth muscle cells, which are relevant to the cellular and molecular events induced by PDGF [
19].
In the present study, we investigated the role of PDGF in the pathogenesis of NPs. There was no significant difference between the PDGF PI values of mucosal layers in all four groups. The PMNC percentages in ethmoid and maxillary sinus, and all cells (PMNCs+MNCs+fibroblasts) PDGF PI of the ethmoid sinus and nasal cavity were higher than the control group. In ethmoid sinus, the PDGF_D_gland PI values decreased in older patients. Chronological ageing alters the fibroblasts metabolism by reducing their lifespans, and capacities to divide and reproduce collagen [
20]. Moreover, the atrophic changes in glands and decreases in fibroblast derived from PDGF may cause decrease of PDGF-gland values in older patients.
As polyp duration gets longer, the PDGF_D_endothelial PI values increased in nasal cavity polyps. Zhou et al. [
21] reported age-dependent increases of PDGF-B expressions in aortic endothelial cells of hypercholesterolemic rats. In our study, the PDGF increase in endothelial cells within the deep layer is similar to Zhou et al.'s study [
21]. Herein, this increase may be related to vascular sclerosis in older patients. In addition, with the increase of tissues nutrition and blood supports, the angioneogenesis may increase due to high PDGF-endothelial levels.
The confounding factors affecting PDGF-PI in layers of mucosa were analyzed by linear regression analysis (backwards):
1. In male polyp patients, the PDGF_D_pv PI increased. It was reported with no influences of age and gender for the growth factor content, including PDGF [
22]. Whereas, Kajizuka et al. [
23] mentioned increased levels of serum PDGF-BB homodimers in males. Thuillier et al. [
24] suggested that PDGF receptor activation is similar to 17 beta-estradiol. By considering Thulier et al.'s study [
24], we would expect PDGF increases amongst females. However, in our study, high PDGF levels in males may be related to the environmental factors exposed more by the males. As a limitation of our study, the male:female ratio was 21:3, and this matter may also affect the results.
2. As Brinkman Index increased, the PDGF_EP_basal PI and PDGF_D_endothelial PI increased, whereas PDGF_SE_pv PI decreased.
3. In ethmoid and maxillary sinuses, the PDGF_SE_pv PI values increased; and in nasal cavity polyps, the PDGF_SE_pv PI values decreased.
4. In older polyps, the PDGF_SE_pv PI values decreased and PDGF_D_endothelial PI increased.
5. As MNC-% increased, the PDGF_EP_basal PI, PDGF_SE_endothelial PI, and PDGF_D_endothelial PI decreased.
6. When the PDGF-PMNC PI increased, the PDGF_D_pv PI decreased, and PDGF_D_endothelial PI increased.
7. As PDGF-MNC PI increased, the PDGF_EP_apical PI, PDGF_SE_endothelial PI, and PDGF_D_endothelial PI decreased.
8. When PDGF-all cells PI increased, PDGF_EP_basal PI and PDGF_D_endothelial PI decreased while the PDGF_D_pv PI increased.
PDGF stimulates chemotaxis of macrophages and polymorphonuclear leukocytes. In fibroblasts and smooth muscle cells, it stimulates both the chemotaxis and mitogenesis. It also stimulates synthesis of fibronectin and hyalurane, and increases the activity of collagenase. A concentration of PDGF in a field shows which cells are capable of responding to PDGF, because different cells are attracted to the medium with different concentrations of PDGF [
4].
In our study, we observed that PDGF was not expressed in PMNCs (
Fig. 3). In all cells group of inflammatory cells, there were PMNCs, MNCs, and additional fibroblasts. Higher PDGF expressions of all cells group as compared to MNCs were due to fibroblasts and higher PDGF expressions within the fibroblasts (
Fig. 3). On the light microscopic examination, in PDGF stained sections, diffuse positivity was detected in both the surface and the mucosal gland EP. PDGF expression was observed mainly around the glands and blood vessels in the subepithelial and deep layers; and, also at superficially located vascular endothelial cells. In polyp tissues, the PDGF expression was less due to smaller number of cells in edematous areas.
In our study, for polyp patients (groups 1-3) with higher MNC percentages and PDGF PIs, endothelial PDGF PI decreased in SE and deep layers of the mucosa, and no significant relations with perivascular PDGF from all layers were detected. However, in patients with higher PDGF-all cells PI values, the perivascular-PDGF was found as higher in deep layer. As we stated that higher PDGF expressions of all cells group as compared to MNCs were due to fibroblasts, the main cells supporting higher perivascular PDGF values in the deep layer of the mucosa were the fibroblasts.
In polyp pathogenesis, the fibroblast-derived PDGF may be more important than MNC-derived PDGF. For patients whose MNC counts and MNC-derived PDGF levels were higher, endothelial PDGF levels were found to decrease and no relation was detected between the perivascular-PDGF and MNCs (count or PDGF-PI). However, in patients with all-cells-PDGF and increased fibroblasts, perivascular PDGF was found higher than the deep layer of the mucosa.
We concluded that PDGF derived from the fibroblasts and the perivascular cells exert its effect on perivascular cells by causing an increase in vascular permeability, migration of inflammatory cells to extracellular area and increments of extracellular edema which might play very important roles in the pathogenesis of sinonasal polyps.
When PDGF-PMNC PI is increased, the PDGF_D_pv PI decreased. In polyp patients, both PMNC-derived PDGF expressions and PMNC derived perivascular PDGF expressions were in a very low state. In addition, as we described above, the PDGF mainly affect perivascular cells, therefore, PMNCs may not have an important role over the PDGF system.
PDGF_SE_pv PI was found higher in ethmoid and maxillary sinuses, and lower in nasal cavity polyps. This difference may be related to oxygen concentrations: PDGF synthesis is often increased in response to external stimuli, such as exposures to low oxygen tension [
25,
26]. In low tissue oxygen levels due to nasal obstruction, the PDGF expression increases higher oxygenization especially in perivascular cells of the nasal stroma. Thereafter, angiogenesis and formation of new blood vessels can occur. It may be considered that PDGF expression increases due to low oxygen levels in nasal obstruction, and this may be one of the mechanisms in the body which increases the tissue oxygenation by increasing blood supplies, the angiogenesis. Due to increased vascular permeability, the increased migration of inflammatory cells to extracellular areaand increased extracellular edema may result in polyp formations.
Ohno et al. [
19] investigated the localization of mRNA and protein of PDGF-B chain (PDGF-B) in NP tissues and bronchial tissues from mild and severe asthmatics by in situ hybridization and immunohistochemistry, respectively. Cells expressing PDGF-B mRNA were found in all nine NP tissues and in bronchial tissues from 2 out of 6 normal subjects, 2 out of 5 mild asthmatics, and all 6 severe asthmatics were examined. The vast majority of cells expressing PDGF-B mRNA were eosinophils in NP and asthmatic bronchial tissues, but no cells expressing PDGF-B mRNA were eosinophils in normal bronchial tissues. The number of cells expressing the genes of severe asthmatic tissues were similar to that in the NP tissues and greater than that in mild asthmatic tissues, which was not significantly greater than that from the normal bronchial tissues.
In our study, versus Ohno et al.'s study [
19], the PDGF expression in PMNCs (including polymorphonuclear leukocytes and eosinophils) was a very low (median, 0.0 in all groups) (
Table 1,
Fig. 3). Our study demonstrated that PDGF expression was provided mainly from MNCs and all cells group (In this group, fibroblast were present with MNCs and PMNCs). The main source for perivascular PDGF was probably the fibroblasts according our data and analysis.
Coste et al. [
27] studied whether the modifications of epithelial differentiation and proliferation being observed in NPs could be related to local secretions of growth factors, among which PDGF could play a key role. In this prospective study, by immunohistochemistry, the proliferating cell nuclear antigen (PCNA, an S-phase cell marker), PDGF, and CD-68 (activated macrophages marker) expression in NP and inferior turbinate mucosa (NM) were assessed from 11 patients. Their data show that PCNA and PDGF expressions are increased in NP EP, while the CD-68 expression is increased in NP EP and lamina propria when compared to NM. Increased local PDGF secretion by numerous activated macrophages could therefore be involved in up-regulation of epithelial cell proliferation in NP. PDGF could also be involved in the pathogenesis of NP via its connective tissues remodelling actions.
In our study, as seen on
Table 1 and
Fig. 1, the PDGF expressions were higher in epithelial and subepithelial layers of lamina propria. In terms of epithelial higher PDGF values, our results were similar to Coste et al.'s study [
27]. Epithelial PDGF expression may also contribute to epithelial permeability, and also act as passage of antigens and irritants to submucosal layers, and this process may also initiate antigen stimulations and cell responses such as mast cells and plasma cells.
Perivascular PDGF was found to be lower at SE layer of the mucosa in smokers and patients with older polyps. Both smoking and longer polyp durations are the factors which cause vascular problems and fibrotic polyps. We assume that possible mechanisms is low in perivascular PDGF PI levels, and the cell migration out of the vascular system may be reduced with less blood vessel formation which allows more fibrotic polyps to develop.
We concluded that PDGF system plays an important role in polyp pathogenesis. Fibroblast-derived PDGF may be more important than MNC-derived PDGF in polyp developing processes. Increased perivascular-PDGF-PI in deep layers of the mucosa causes vascular permeability; the increased migration of inflammatory cells to extracellular area and increased extracellular edema may result in sinonasal polyp formations. Tissues oxygenization may be important for initiating the PDGF release system. Thus, nasal obstruction should be medically treated earlier, and, if necessary, by surgical approaches.
Go to :











 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download