Abstract
Progress in head and neck cancer (HNC) therapies has improved tumor response, loco-regional control, and survival. However, treatment intensification also increases early and late toxicities. Dysphagia is an underestimated symptom in HNC patients. Impairment of swallowing process could cause malnutrition, dehydration, aspiration, and pneumonia. A comprehensive literature review finalized in May 2012 included searches of electronic databases (Medline, Embase, and CAB abstracts) and scientific societies meetings materials (American Society of Clinical Oncology, Associazione Italiana Radioterapia Oncologica, Associazione Italiana di Oncologia Cervico-Cefalica, American Head and Neck Society, and European Society for Medical Oncology). Hand-searches of HNC journals and reference lists were carried out. Approximately one-third of dysphagia patients developed pneumonia requiring treatment. Aspiration pneumonia associated mortality ranged from 20% to 65%. Unidentified dysphagia caused significant morbidity, increased mortality, and decreased the quality of life. In this review we underline definition, causes, predictive factors of dysphagia and report on pretreatment and on-treatment evaluation, suggesting some key points to avoid underestimation. A multi-parameter assessment of swallowing problems may allow an earlier diagnosis. An appropriate evaluation might lead to a better treatment of both symptoms and cancer.
Progress in head and neck cancer (HNC) treatments has improved tumour response and loco-regional control rates. However, despite improved diagnostic and therapeutic approaches, mortality remains high [1,2].
Intensification of treatment with chemoradiotherapy (CRT) or altered fractionation radiotherapy (RT) is associated with improved outcome, but causes severe early and late mucosal and pharyngeal toxicities. Oropharyngeal dysphagia is an underestimated symptom in HNC patients [3,4].
Frequent causes of dysphagia in this population include neurological and neuromuscular impairment, and structural and iatrogenic causes. Dysphagia should not be neglected, as it can profoundly diminish the quality of life (QoL) [5]. The resulting impaired swallowing can cause malnutrition and dehydration, and might lead to aspiration pneumonia. Swallowing disorders are often predictable, depending on both tumor associated structures and treatment modalities. A correct pretreatment selection for patients at highest risk for dysphagia could optimize functional and therapeutic results [6,7]. A multidimensional approach should consider treatment targets and acute and late toxicities. For most patients the highest priority is cure, therefore considerations about late treatment-related toxicities should not prevent the use of proven aggressive therapy, provided that the balance between toxicity and probability of cure has been discussed and accepted by the patient.
Acute dysphagia is often considered of less concern due to its transient nature. Nevertheless, it is a well recognized cause of malnutrition that leads to significant morbidity, higher mortality, and decreased QoL [8,9]. Furthermore enhanced acute toxicity may amplify late-effects such as fibrosis and lymphedema resulting in increased dysphagia [10].
It is important that clinicians are aware of correlations between acute and late toxicities, and are capable of recognizing patients at risk for severe acute dysphagia, to reduce late dysphagia, prevent malnutrition, and provide aspiration, with the goal of providing the proper supportive care for these patients.
Adequate diagnosis and care during the treatment may increase compliance with the therapeutic protocol with a complete dose delivery of chemotherapy (CT) and RT. With this aim, we presently reviewed the relevant literature in terms: 1) definition, physiology and causes, 2) pretreatment evaluation of swallowing disorders and predictive factors, and 3) evaluation and support measures during treatment, and offer conclusions and recommendations.
A comprehensive literature review was finalized in May 2012. Electronic databases (Medline, Embase, and CAB abstracts) and scientific societies meeting materials (American Society of Clinical Oncology, Associazione Italiana Radioterapia Oncologica, Associazione Italiana di Oncologia Cervico-Cefalica, American Head and Neck Society, and European Society for Medical Oncology) were searched with the date parameters of January 1990 through May 2012. The decision concerning this range was made on the basis of the publication dates of the most important research clinical trials, investigating dysphagia in acute and late toxicities of HNC treatment.
Electronic search results were supplemented with hand searching of selected reviews, expert consensus meeting notes, and reference lists from selected articles. The literature search was limited to articles in English concerned with human patients. Medical subject headings (MeSH) terms and keywords used in the search were dysphagia, malnutrition, weight loss, head and neck cancer, chemoradiotherapy, acute toxicity, and late toxicity.
Dysphagia is defined as the difficulty or impossibility to swallow liquids, food, or medication. Dysphagia can occur during the oropharyngeal or oesophageal phase of swallowing. Normal swallowing is a complex and well-coordinated process, which requires neural control regulated by interactions between cortical centres in both hemispheres, the swallowing centre in the brainstem, cranial nerves (V, VII, IX sensory, IX motor, X, and XII), and pharyngeal receptors for touch, pressure, chemical stimuli, and water. Normal swallowing comprises four phases: oral preparation, oral, pharyngeal, and oesophageal [11].
During the oral preparatory phase of swallowing, the food is ground and mixed with saliva to form a bolus. In the oral phase, the bolus is transported to the pharynx. The swallowing reflex is triggered during the pharyngeal phase, resulting in closure of the larynx to prevent aspiration, contraction of the pharyngeal constrictors from superior to inferior, laryngeal elevation and epiglottis inversion, and relaxation of the crico-pharyngeus to allow the food bolus to pass into the oesophagus. During the final phase, the peristalsis of the oesophageal muscles results in movement of the bolus into the stomach. Deregulation in any of these functions can result in dysphagia. Additionally, swallowing and neck movement require that the pharyngeal structures and carotid sheath move easily relative to the spine and prevertebral space. The pharynx is essentially a muscular tube suspended from the skull base. The fat in the retropharyngeal and para-pharyngeal spaces allow for this necessary movement and pharyngeal expansion as well [11]. Penetration is defined as the passage of material into the larynx that does not pass below the vocal folds. The amount of material, depth of penetration, and whether all or a portion is subsequently expelled are potentially critical variables and deserve study, but are not part of the definition. Aspiration is defined as passage of material below the level of the vocal folds [12].
Causes of dysphagia include different alterations of the swallowing process that can interfere with physiological functions in each step of these described. Damage at anatomical structures or neurological damage may hinder normal physiology. Most common alterations include structural incontinence of the oral cavity; incorrect movement of the supraglottic larynx, epiglottis, and vestibule; reduced pharyngeal peristalsis; alterations of prevertebral space; and other muscular and neurological dysfunctions. All these types of damage contribute differently and to varying degrees to dysphagia after HNC treatment (Table 1) [4].
Swallowing disorders may cause aspiration that is usually prevented by an intact cough reflex. Aspiration causes can be divided to those occurring before, during, and after swallowing (Table 2) [4]. The incidence of silent aspiration varies from 9% to 18.5% at diagnosis and from 22% to 60% after specific treatment [13-15]. Aspiration due to neurological causes typically occurs before or during swallowing, while in HNC patients it occurs after swallowing due to the entry of excessive residue into the larynx through damaged regions. Neurological and structural impairment can cause distinctive swallowing problems.
In HNC patients, structural impairment generally prevails even if both these problems can be contemporaneous, as a consequence of structural damage involving nerve or muscles or related to the consumption of certain medications. Anticholinergic drugs, steroids, asthma medications, vasoconstrictors, or expectorants can cause xerostomia. Antidepressants, anti-anxiety agents, antipsychotic, sedatives, and hypnotic agents can depress the central nervous system. Some antipsychotics may also cause extra-pyramidal effects with facial and mouth dyskinesias. Penicillamine or antibiotics like aminoglycosides and erythromycin may block the neuromuscular junction. Corticosteroids or lipid lowering agents can cause drug-induced myopathy [16].
Evaluation of swallowing disorders in naïve HNC patients is complex and requires a multi-team collaborative effort involving head and neck surgeons, speech pathologists, radiation oncologists, medical oncologists, radiologists, and nutritionists. All patients at risk should be screened by a multimetric model in which more than one parameter indicates dysphagia. Murphy's trigger symptoms, excessive chewing, drooling, and complaint of food sticking in the throat are suggestive of dysphagia (Table 3) [17]. Of particular concern are symptoms that indicate potential aspiration, including coughing or clearing the throat before, during, or after eating. If patients develop any of these symptoms, an immediate referral for assessment by a Speech Language Pathologist should be considered. Patients with significant aspiration risk and those who need enteral/parenteral nutrition should be identified and enrolled in a program that includes education and swallowing therapy. Adequate and safe nutrition should also be guaranteed. Patients with silent aspiration often subconsciously reduce their oral intake and lose weight; this finding alone should lead to instrumental assessment [18]. Rosen et al. [19] reported in a prospective study on newly diagnosed HNC patients that experienced clinicians (otolaryngologists and speech pathologists) correctly predicted only six of 11 patients who actually aspirated on videofluoroscopy. The difficulty in predicting aspiration was attributed to the absence of the cough reflex in some patients.
Instrumental assessment of swallowing in HNC patients provides useful information about both the structure and function of this mechanism. Two procedures are usually performed: video-fluoroscopic modified barium swallow (VMBS) and fiberoptic endoscopic evaluation (FEES).
VMBS is a video-fluoroscopic examination that allows evaluation of oral and pharyngeal function by successive records of images, while FEES is a fiber-optic endoscopic examination (which avoids radiation exposure) that allows an excellent visualization of anatomy, including postsurgical or postradiation modifications or lesions. Both VMBS and FEES identify disorders that impair swallowing, cause aspiration, and increasing the risk for pneumonia. Additionally, they provide an evaluation of a patient's ability to maintain nutrition and hydration. Standardized protocols have been established for VMBS that test swallowing capacity using contrast containing food boluses of varying sizes and consistencies, thus allowing a Speech Language Pathologist to make dietary recommendations for patients with impaired swallowing. If abnormalities are identified, various compensatory measures including postural techniques, increased sensory input, and voluntary swallowing manoeuvres can be assessed for efficacy [11].
The penetration-aspiration scale has been developed to allow objective reports of penetration and aspiration events. The 8-point scale provides reliable quantification of selected penetration and aspiration events observed during video-fluoroscopic swallowing evaluations. Other systems can be used to specify the amount and timing of penetration and aspiration events. These scoring systems do not substitute for other perceptual measures of swallowing tested with VMBS and FEES. However, the use of these scales permits a numeric quantification of dysphagia, facilitating accurate communication among clinicians.
In clinical practice, FEES is more convenient and less expensive than VMBS, and can be performed repeatedly [20]. Thus, a large number of dysphagic patients may be evaluated with FEES, while VMBS may be used less frequently and in selected cases. However, a prospective, randomized trial of 126 patients assigned to FEES or VMBS demonstrated no advantage of either technique in predicting aspiration pneumonia in patients with dysphagia [21]. Thus, the two instrumental assessments seem to be equivalent and complementary. VMBS allows evaluations of the entire upper digestive system including oral phase deficits, while FEES seems to be an adequate test for evaluation of the pharyngeal phase of swallowing [22].
Instrumental examinations only assess the structures and dynamics of the swallowing process, and do not assess the influence of the swallowing problems on a patient's overall QoL (i.e., personal perception of well-being). HNC and its treatment can affect both disease-specific health-related QoL (HRQoL), such as salivary and swallowing functions, and the general domains of HRQoL, such as physical, mental, and social health [8]. Recently, Langendijk et al. [22] advocated a simple measure designated the total dysphagia risk score (TDRS) to predict swallowing dysfunction after curative RT for HNC. TDRS is a summation of the following risk points: T-classification (T3, 4 points; T4, 4 points), neck irradiation (bilateral neck irradiation, 9 points), weight loss (1%-10%, 5 points; >10%, 7 points), primary tumour site (oropharynx, 7 points; nasopharynx, 9 points) and treatment modality (accelerated RT, 6 points; concomitant CT, 5 points). The authors also reported that this predictive model could also be adapted for acute morbidity. In a retrospective study with 47 patients, Kowai et al. [23] observed swallowing dysfunction (as Radiation Therapy Oncology Group [RTOG] grade 2 or higher) in 27 patients (57%; P<0.001). The cut-off value of the TDRS was set at 18 (sensitivity, 0.81; specificity, 0.85). Prediction of severe (grade 3) acute swallowing dysfunction was similarly obtained. The authors concluded that the TDRS is a useful tool to predict acute swallowing dysfunction induced by CRT for HNC.
Despite the plethora of available indices for cancer in general, few measurement tools are specific to HNC. Most of those HNC-specific tools measure QoL and few measure the common comorbidities of speech and swallowing. As a result, there is a gap of outcome measures specific to speech and swallowing that requires addressing [24].
Van der Molen [14] conducted a systematic review of the literature and found only a few studies combined VMBS examinations with QoL questionnaires. Some authors showed the utility of monitoring dysphagia problems that afflict these patients in their multidimensional aspects: pre- and posttreatment dysfunction, objective instrumental and clinical (symptom and sign) rated-assessment, and subjective clinician-rated and patient-rated assessments of swallowing abnormalities (European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 [EORTC QLQ-C30] and the EORTC QLQ-H&N35) [13,24]. Several pretreatment functional problems identified either by instrumental assessment (e.g., postswallowing residue) or clinician assessment is not perceived by patients; 30% have silent penetration/aspiration [14].
Recently Christianen et al. [6] eported a large multicentre prospective cohort study that evaluated toxicity and HRQoL prior to, during, and at regular intervals after curative CRT. The authors investigated dose volume histogram parameters and pretreatment factors to establish those predictive of radiation-induced swallowing dysfunction.
T and N stage, primary site, type of treatment, extension of treated region (volume of tissue and anatomic structures), patient characteristics (baseline swallowing function, performance status [PS], smoking and alcohol abuse, age, lean mass, gender) predict the risk of acute and late dysphagia.
All treatment modalities, whether involving surgery or organ sparing protocols, and CRT result in swallowing problems along with aspiration. Therefore, we can classify factors predictive of dysphagia as patient-related, tumor-related, and treatment-related [18,25]. Patient characteristics such as baseline swallowing function, PS, smoking and alcohol abuse, age, lean mass, and gender predict the risk of dysphagia [13]. Advanced T and N stage are associated with worst swallowing impairment [22].
Whether a different primary tumor site is better related with frequency and severity of acute and late dysphagia is contentious. Aspiration is most frequent in hypopharynx or larynx cancer patients before treatment; the worst base-line function could account for higher rate of swallow impairment in this subset of patients [26].
Logemann [27] in a consecutive series of 53 VMBS reported that pressure generation during swallowing and airway protection are the most frequent disorders observed (reduced tongue base retraction and reduced tongue strength). A more frequent reduction in tongue base movement was described in patients with oropharynx and larynx cancer. Since the tongue base lies between the oropharynx and larynx, it is likely that the tongue base receives the maximal radiation dose when these areas are irradiated. However, the study was limited by the small number of patients for each site of the primary tumor.
In a similar study, Frowen et al. [28] found that patients with hypopharyngeal tumors had significantly worse swallowing, compared with either oropharyngeal or laryngeal tumors, for all instrumental measures (P=0.001 to P=0.042), except the penetration/aspiration of liquids. At 6 months posttreatment, patients with hypopharyngeal tumors were still experiencing a moderatesevere or moderate degree of activity limitation. For 50% of these patients, enteral nutrition was still required.
Patients with oropharyngeal tumors reportedly have significantly worse activity limitation for semisolids than patients with laryngeal tumours (P=0.01), particularly at 3 months posttreatment (43% for mild limitation vs. 73 for no limitation) [29]. After 6 months these differences were reduced (74% vs. 86%) with only a transient risk of airway penetration. Moreover, patients with laryngeal cancer are thought to be less at risk for weight loss and reduced food intake than patients with other primary HNC [29].
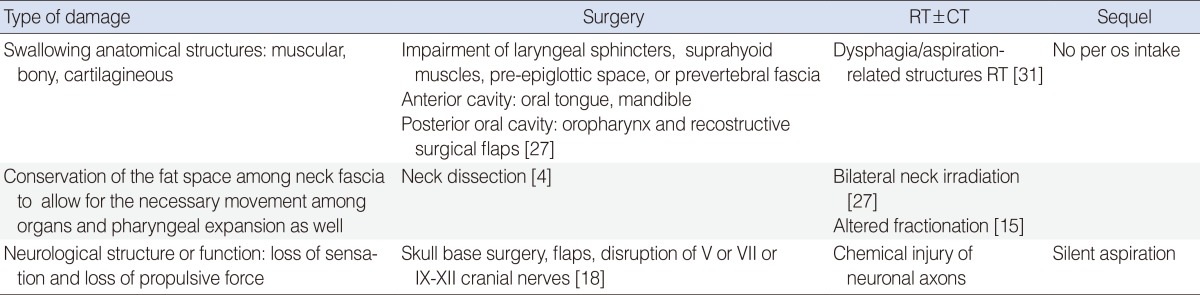
The type of treatment (organ anatomic preservation vs. surgery, demolitive vs. partial surgery, concurrent CRT vs. RT) and the extension of treated region (volume of tissue and anatomic structures) results in different severity of this sequelae [30]. Shune et al. [26] recently reported the association between severity of dysphagia and survival defining risk factors: advanced stage, older age, female sex and hypo-pharyngeal tumors. Table 4 summarizes three causes that, in our opinion, most influence dysphagia [4,15,18,27,31].
Surgery in HNC patients may cause dysphagia by damage/resection of muscular, bony, cartilaginous, or nervous structures (swallowing anatomical structures and neurological structure) as well as by neck fascia removal. The severity of the swallowing deficit is dependent on the size and location of the lesion, and the degree and extent of surgical resection [32]. However, Miller and Groher [33] proposed that the removal of less than 50% of a structure involved with swallowing will not interfere or seriously influence swallowing function.
The importance of the anatomical region of excision has been highlighted by several reports. The size of the lesion excised is less prognostic than the excised area. Therefore, dysphagia can be accurately predicted for some surgeries, such as the base of the tongue and arytenoid cartilage resections [34]. Even though the introduction of robotic surgery has improved outcomes, several reconstructions achieve an aesthetic, but not functional, objective (tissue flaps have no motor function resulting in the loss of propulsive force). Surgical complications including nerve function interruption may affect swallowing function. Furthermore, neck dissection significantly increases aspiration as well as gastrostomy tube dependence [32]. Edema, pain, scarring, and nerve injury due to neck dissection are all potential causes in the pathogenesis of swallowing dysfunction [11].
Despite the fact that modern RT protocols are designed to spare normal tissue and preserve structure and function, dysphagia remains a potentially life-threatening occurrence in HNC patients treated with RT or CRT. Irradiation of swallowing structures and altered dose fractionation contributes to worsened dysphagia. Eisbruch et al. [31] identified dysphagia/aspiration-related structures (DARSs) whose treatment-related damage can lead to swallowing dysfunction. DARSs include the pharyngeal constrictor muscles (PCM), supraglottic larynx, and glottic larynx.
Bilateral neck RT has been identified as a prognostic factor for dysphagia and weight loss in early stage laryngeal cancer (T1/T2), although it is difficult to discern whether a larger tumor (high stage) or treatment protocols per se most affect dysphagia. Langius et al. [29] reported that RT on cervical level II-IV lymph nodes has the most negative effect, likely due to irradiation of major salivary glands with consequently xerostomia, acute dysphagia, and weight loss. Accelerated RT can worsen acute dysphagia compared to conventional fractionation, as shown in the analysis of data from the DAHANCA trial [35]. Severe dysphagia, defined as liquid food only or worse, and no intake per os (grade 3 or 4) occurred in 47% and 38% of patients receiving accelerated or conventional RT, respectively (P=0.001). Significant independent factors for severe acute dysphagia were T3-T4 tumors, N-positive and non-glottic cancer, age >62 years, baseline dysphagia >0, and accelerated RT. The use of intensity-modulated radiation therapy (IMRT) minimizes radiation to DARS resulting in improved swallowing outcomes. The main gain in dysphagia-sparing IMRT is obtained when medial retropharyngeal nodes are spared, assuming they are at low risk for failure [31].
Levendag et al. [36] in a cohort of 81 patients demonstrated a steep dose-effect relationship with an increase in the probability of dysphagia of 19% with every additional 10 Gy after 55 Gy for pharyngeal superior constrictor muscle and mean constrictor muscle. The volume of RT-treated swallowing structures is a prognostic factor. In the Trans Tasman Radiation Oncology Group (TROG) 91.01 trial, the volume of irradiated pharynx (which included the mucosa and underlying pharyngeal constrictor muscles) was correlated to the probability of requiring enteral feeding [37].
A correlation between food consistency and anatomical cause of dysphagia was reported by Christianen et al. [6]. Damage of the superior PCM is responsible for solid food dysphagia. Laryngeal elevation and cricopharyngeal opening is necessary for pharyngeal clearance, which may lead to patient self-restriction in the amount and viscosity of food taken. In combination with inadequate airway closure at the supraglottic larynx, this could lead to aspiration. This knowledge permits a correlation among some unexplained weight loss in early stage HNC and the specific swallowing structure dysfunction.
Although CRT improves locoregional control and overall survival, and allows for organ preservation, it increases toxicities compared with RT alone [4]. The most common acute grade 3 to 4 complications (leucopenia, anemia, mucositis, and dysphagia) are increased 14% to 43% over RT [38]. CRT often results in higher rates of swallowing difficulty. Furthermore, side-effects like nausea, vomiting, neutropenia, generalized weakness, and fatigue can occur in acute dysphagia and malnutrition. All patients receiving CRT report some grade of mucositis. Drugs commonly used in CRT are anti-metabolites, taxans and platin salts. Anti-metabolites like methotrexate and 5-fluorouracil seem to be the drugs that are most associated with mucositis. Taxans are associated with allergy and peripheral neurotoxicity, while platins are more associated with hematological toxicities and dysgeusia [38,39].
Concomitant CT emerged as the strongest independent factor correlated with acute morbidity in several studies [17,38,39]. When CT is associated with RT, the critical dose to swallowing structure is lower. These differences seem related to acute mucositis and its consequential effect on pharyngeal tissue. Chemoradiation regimens that do not differ markedly in the rate and severity of the acute mucositis seem to cause similar types and rates of swallowing abnormalities than RT alone [15]. In a population of platinum-based CRT patients, the 5-year actuarial rates of overall late RTOG/EORTC grade 3 and grade 4 toxicity were 52% and 25%, respectively. Radiologic evaluation after a median follow-up of 44 months demonstrated impaired swallowing in 57% of the patients, including 23% with silent aspiration. Subjective assessment using a systematic scoring system indicated normalcy of diet in only 15.6% of the patients [15].
Data from randomized trials have added further evidence. In the RTOG 91-11 randomized trial, the incidence of severe (grade 3, 4) stomatitis and dysphagia increased with CT, respectively, from 24% and 19% (RT alone) to 43% and 35% (concomitant CRT), although skin effects were not altered (7% vs. 9%) [40]. In the EORTC 22931 trial, the incidence of severe functional mucosal effects increased with CRT from 21% to 41% [41]. In the RTOG 95-01 trial, concomitant CT had an adverse effect on severe mucositis (from 18% to 30%) and dysphagia (from 15% to 25%), but not on skin morbidity (10% vs. 7%) [42]. In a nonrandomized comparison of patients treated at a single centre in prospective phase I and II trials of concomitant CT-IMRT (n=85) and the phase III trial of IMRT vs. conventional RT (PARSPORT) (n=82), G3 dysphagia was recorded prospectively [43]. Feng et al. [3] recently showed benefits from efforts to spare the swallowing structures in chemo-IMRT treated patients reporting a dose-volume aspiration effect for the PCM. Benefit to maximized superior constrictors was involved. The dose-volume effect relationships for the swallowing structures may depend on the intensity of the CRT regimen. Strictures were not observed in patients receiving mean pharyngeal constrictors doses exceeding 66 Gy. These relationships support the hypothesis that a lower dose to the swallowing structures may reduce the prevalence and severity of dysphagia. However, the available data do not yet prove this hypothesis because they do not establish a cause-effect association [3].
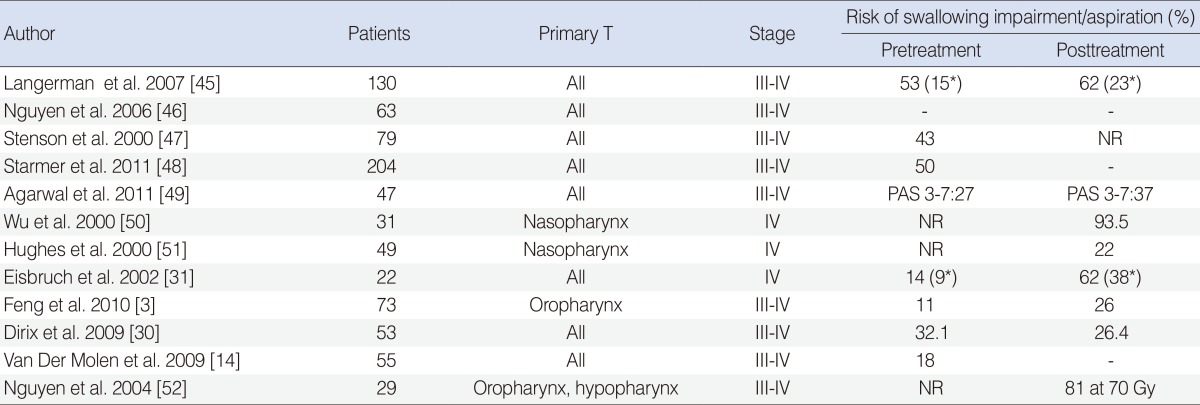
Additionally, it is not known if the combination of epidermal growth factor receptor (EGFR) inhibitors and RT results in lower rates of swallowing disability. In 2006, Bonner et al. [44] published the results of a phase III trial that showed the efficacy and safety of the addition of cetuximab, a monoclonal antibody developed to inhibit the EGFR pathway. Cetuximab enhanced the effects of RT and improved survival at 5 years in patients with advanced HNC. Skin toxicity was the most frequent side effect of the cetuximab-RT regimen. However, more severe mucositis and acute dysphagia than occurring with RT alone has been reported. Table 5 provides a summary of the most impressive trials evaluating dysphagia during HNC treatment [14,30,31,45-52].
Dysphagia can directly result in decreased eating, malnutrition, and weight loss [26]. Severe unintentional weight loss occurs in 5% to 71% of patients with HNC and averages 6% to 12% of pretreatment body weight [10]. Weight loss can be attributed to energy imbalance consisted of decreased energy intake from reduced food consumption and/or increased energy expenditure from altered metabolic rate.
Weight loss is associated with a significantly lower survival rate and is an independent predictor for mortality in patients with stage III and IV tumors. Body weight loss also causes RT dose problems. The risk of delivering an inadequate radiation dose to the target volume and critical structures may arise if coordinated re-planning is not performed during the course of the therapy, especially when using highly conformal methods [23]. Nutrition in HNC patients with a high risk of dysphagia is still debatable. On the one hand, systematic use of the percutaneous endoscopic gastrostomy tube (PEG) may avoid weight loss [53]. On the other hand, it may expose a significant proportion of patients to needless cost and risks of tube placement [54]. Furthermore, the potential benefit from a wait-and-see procedure with PEG insertion is supported by the findings of complications and prolonged dysphagia in patients whose treatment utilizes a PEG. In patients that do have a high risk of weight loss a short period of parenteral nutrition may be adequate [55]. Enteral nutritional treatment can be indicated when weight loss exceeds 5% of the patient initial weight [56], whereas other authors advocate that enteral therapy should begin before RT treatment [57]. It is unclear whether dietary counselling or nutritional support actually increases lean mass in HNC with dysphagia, with dietician evaluation at baseline recommended [58,59].
A secondary analysis of RTOG 90-03 reported that nutritional support before RT is associated with poorer treatment outcome [60]. Indeed, patients on nutritional support delivered before treatment had significantly less weight loss and grade ≥3 mucositis. However, surprisingly they had worse 5-year loco-regional control (LRC). These conclusions did not come from a pre-established analysis, limiting their power.
Prevention and treatment of mucositis and swallowing-induced pain are areas of great interest, but a golden standard is still not available. In a majority of patients, pain (tumor- and treatment-related) can be severe and require major analgesics. Both pain and opioids can contribute to decreased dietary intake and the latter increases gastro-intestinal motility alterations [61].
A recent Cochrane review [62] reported that retrospective studies have revealed complications including laryngeal irritation and persistent gastro-oesophageal reflux in patients fed with a nasal gastric tube (NGT). Furthermore, use of a NGT may increase patient discomfort, and increase the risk of tube displacement and blockage compared to use of a PEG. PEG feeding may be the preferred method in patients with radiation-induced oral and esophageal mucositis. Potential advantages of PEG over NGT include enhanced mobility, improved QoL, and consumption of higher caloric food. According to Nugent et al. [62], 2010 PEG should be recommend to all patients before treatment, in view of its beneficial effect on QoL. Conversely, prolonged enteral nutrition status is directly correlated with worse swallowing outcomes and increased risk for dysphagia. Atrophy of pharyngeal and tongue-base musculature and increased pharyngeal fibrosis can result both from general non-use of swallowing musculature and from a marked decrease in patient swallowing (volitional or spontaneous) [26].
We suggest elective use of PEG to reduce swallowing difficulties, as secondary consequences of prolonged enteral status. Timely identification of the subgroup of patients with dysphagia or with a risk of developing severe dysphagia that will require a PEG before or during treatment is critical to maximize benefits [63]. The TDRS may serve as an index to enable selection of appropriate candidates for prophylactic PEG placement [15]. However, Mangar et al. [64] showed that some clinical parameters, such as tumor site, PS 2-3, older age, low body mass index, and serum albumin predict nutritional deficit. Treatment for oropharyngeal dysphagia is typically quite different than that for esophageal dysfunction. While there are some drugs and surgical procedures available to improve function of the esophageal swallowing process, in the pharynx there are not the same possibilities for part of the process. Rehabilitation includes behavioural changes, such as posture, sensory stimulations, swallow manoeuvres, voluntary controls exerted over the swallow, and/or changes in diet [11].
Emerging data indicate that early intervention with swallowing exercises may improve dysphagia, whereas delayed swallowing therapy achieves only minor benefit [65]. Other data suggest that function at 6 months predicts long-term function [66]. It therefore seems reasonable to aim for maximal swallowing recovery by 6 months post-CRT, but randomized trials are necessary to confirm these findings. Pharmacologic interventions, such as amifostine and keratinocyte growth factor, may reduce toxicity and are showing promise, but are of secondary importance to good radiation technique and support of the health care team [37]. HRQoL questionnaires evaluating dysphagia in the literature include the SWAL-QoL, the MD Anderson Dysphagia Inventory, and the Deglutition Handicap Index [66,67].
Dysphagia is an increasingly recognized problem in the treatment of HNC. It affects QoL and survival. To ensure adequate therapies for RT and/or CRT candidates, a pretreatment evaluation of swallowing function and nutritional status is needed. A new standard of multidisciplinary approach in HNC should include routine diagnostic swallowing assessments and therapeutic interventions before, during, and after therapy. Data collected in the present systematic literature review indicate that surgery and RT or CRT can impair swallowing. Swallowing and neck movement require that pharyngeal structures, visceral fascia, and sheath move easily relative to the spine and prevertebral space. Surgical or RT fibrosis and anatomic concerns hinder this necessary movement and pharyngeal expansion as well. Dysphagia has been not adequately considered during HNC treatment plans. However, in the past several years there has been a growing interest around the major common sequelae of surgery and CRT. Understanding of the pathophysiology through the identification of DARS (muscles, glottic and supraglottic larynx, nerves) may allow radiation oncologists to reduce the dose delivered to the swallowing organs. At the same time, ear/nose/throat specialists should avoid aggressive surgery when it is not needed to improve survival outcomes. Dysphagia evaluation should help physicians to determine appropriate cancer therapy, increase patient compliance, and provide adequate posttreatment care. Immediate treatment of dysphagia will increase adherence to treatment protocol, while nutritional support will avoid critical weight loss. Additionally, awareness of dysphagia will also help pain management. For instance, starting intensive rehabilitation with pretreatment swallowing exercises improves posttreatment swallowing function. In Table 5, we summarize some helpful recommendations for early diagnosis and as a guide for the multidisciplinary team.
A multi-parameter assessment of dysphagia that considers the objective-instrumental examinations (e.g., residue research), patient-rated assessment (e.g., pain), and clinician-rated assessment (e.g., weight loss) of swallowing problems can allow for a better diagnosis, based on a better understanding of symptoms. This allows, on one hand, a proper prediction of late dysphagia and, on the other hand, accurate supporting care during the treatment. The diffusion of an adequate HRQoL questionnaire could further contribute to enhanced information from the patients' point of view.
References
1. Argiris A, Li Y, Forastiere A. Prognostic factors and long-term survivorship in patients with recurrent or metastatic carcinoma of the head and neck. Cancer. 2004; 11. 101(10):2222–2229. PMID: 15452834.

2. Pignon JP, le Maitre A, Maillard E, Bourhis J. MACH-NC Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009; 7. 92(1):4–14. PMID: 19446902.

3. Feng FY, Kim HM, Lyden TH, Haxer MJ, Worden FP, Feng M, et al. Intensity-modulated chemoradiotherapy aiming to reduce dysphagia in patients with oropharyngeal cancer: clinical and functional results. J Clin Oncol. 2010; 6. 28(16):2732–2738. PMID: 20421546.

4. Russi EG, Corvo R, Merlotti A, Alterio D, Franco P, Pergolizzi S, et al. Swallowing dysfunction in head and neck cancer patients treated by radiotherapy: review and recommendations of the supportive task group of the Italian Association of Radiation Oncology. Cancer Treat Rev. 2012; 12. 38(8):1033–1049. PMID: 22542950.

5. Pikus L, Levine MS, Yang YX, Rubesin SE, Katzka DA, Laufer I, et al. Videofluoroscopic studies of swallowing dysfunction and the relative risk of pneumonia. AJR Am J Roentgenol. 2003; 6. 180(6):1613–1616. PMID: 12760930.

6. Christianen ME, Schilstra C, Beetz I, Muijs CT, Chouvalova O, Burlage FR, et al. Predictive modelling for swallowing dysfunction after primary (chemo)radiation: results of a prospective observational study. Radiother Oncol. 2012; 10. 105(1):107–114. PMID: 21907437.

7. Eisbruch A, Kim HM, Feng FY, Lyden TH, Haxer MJ, Feng M, et al. Chemo-IMRT of oropharyngeal cancer aiming to reduce dysphagia: swallowing organs late complication probabilities and dosimetric correlates. Int J Radiat Oncol Biol Phys. 2011; 11. 81(3):e93–e99. PMID: 21592678.

8. Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, Leemans CR, Aaronson NK, Slotman BJ. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008; 8. 26(22):3770–3776. PMID: 18669465.

9. Nguyen NP, Moltz CC, Frank C, Karlsson U, Smith HJ, Nguyen PD, et al. Severity and duration of chronic dysphagiafollowing treatment for head and neck cancer. Anticancer Res. 2005; Jul-Aug. 25(4):2929–2934. PMID: 16080546.
10. Trotti A, Bellm LA, Epstein JB, Frame D, Fuchs HJ, Gwede CK, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 2003; 3. 66(3):253–262. PMID: 12742264.

11. Logemann JA, Larsen K. Oropharyngeal dysphagia: pathophysiology and diagnosis for the anniversary issue of Diseases of the Esophagus. Dis Esophagus. 2012; 5. 25(4):299–304. PMID: 21595782.

12. Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996; 11(2):93–98. PMID: 8721066.

13. Eisbruch A, Lyden T, Bradford CR, Dawson LA, Haxer MJ, Miller AE, et al. Objective assessment of swallowing dysfunction and aspiration after radiation concurrent with chemotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2002; 5. 53(1):23–28. PMID: 12007937.

14. van der Molen L, van Rossum MA, Burkhead LM, Smeele LE, Hilgers FJ. Functional outcomes and rehabilitation strategies in patients treated with chemoradiotherapy for advanced head and neck cancer: a systematic review. Eur Arch Otorhinolaryngol. 2009; 6. 266(6):889–900. PMID: 18825400.

15. Rutten H, Pop LA, Janssens GO, Takes RP, Knuijt S, Rooijakkers AF, et al. Long-term outcome and morbidity after treatment with accelerated radiotherapy and weekly cisplatin for locally advanced head-and-neck cancer: results of a multidisciplinary late morbidity clinic. Int J Radiat Oncol Biol Phys. 2011; 11. 81(4):923–929. PMID: 21095074.
16. Wieseke A, Bantz D, Siktberg L, Dillard N. Assessment and early diagnosis of dysphagia. Geriatr Nurs. 2008; Nov-Dec. 29(6):376–383. PMID: 19064135.

17. Murphy BA, Gilbert J. Dysphagia in head and neck cancer patients treated with radiation: assessment, sequelae, and rehabilitation. Semin Radiat Oncol. 2009; 1. 19(1):35–42. PMID: 19028344.

18. Pauloski BR, Rademaker AW, Logemann JA, Lazarus CL, Newman L, Hamner A, et al. Swallow function and perception of dysphagia in patients with head and neck cancer. Head Neck. 2002; 6. 24(6):555–565. PMID: 12112553.

19. Rosen A, Rhee TH, Kaufman R. Prediction of aspiration in patients with newly diagnosed untreated advanced head and neck cancer. Arch Otolaryngol Head Neck Surg. 2001; 8. 127(8):975–979. PMID: 11493209.

20. Martin-Harris B, Michel Y, Castell DO. Physiologic model of oropharyngeal swallowing revisited. Otolaryngol Head Neck Surg. 2005; 8. 133(2):234–240. PMID: 16087021.

21. Aviv JE. Prospective, randomized outcome study of endoscopy versus modified barium swallow in patients with dysphagia. Laryngoscope. 2000; 4. 110(4):563–574. PMID: 10764000.

22. Langendijk JA, Doornaert P, Rietveld DH, Verdonck-de Leeuw IM, Leemans CR, et al. A predictive model for swallowing dysfunction after curative radiotherapy in head and neck cancer. Radiother Oncol. 2009; 2. 90(2):189–195. PMID: 19167120.

23. Koiwai K, Shikama N, Sasaki S, Shinoda A, Kadoya M. Validation of the Total Dysphagia Risk Score (TDRS) as a predictive measure for acute swallowing dysfunction induced by chemoradiotherapy for head and neck cancers. Radiother Oncol. 2010; 10. 97(1):132–135. PMID: 20817288.

24. Martino R, Ringash J. Evaluation of quality of life and organ function in head and neck squamous cell carcinoma. Hematol Oncol Clin North Am. 2008; 12. 22(6):1239–1256. PMID: 19010271.

25. Starmer H, Sanguineti G, Marur S, Gourin CG. Multidisciplinary head and neck cancer clinic and adherence with speech pathology. Laryngoscope. 2011; 10. 121(10):2131–2135. PMID: 21826674.

26. Shune SE, Karnell LH, Karnell MP, Van Daele DJ, Funk GF. Association between severity of dysphagia and survival in patients with head and neck cancer. Head Neck. 2012; 6. 34(6):776–784. PMID: 22127835.

27. Logemann JA. Update on clinical trials in Dysphagia. Dysphagia. 2006; 4. 21(2):116–120. PMID: 16685468.

28. Frowen J, Cotton S, Corry J, Perry A. Impact of demographics, tumor characteristics, and treatment factors on swallowing after (chemo) radiotherapy for head and neck cancer. Head Neck. 2010; 4. 32(4):513–528. PMID: 19691115.
29. Langius JA, Doornaert P, Spreeuwenberg MD, Langendijk JA, Leemans CR, van Bokhorst-de van der Schueren MA. Radiotherapy on the neck nodes predicts severe weight loss in patients with early stage laryngeal cancer. Radiother Oncol. 2010; 10. 97(1):80–85. PMID: 20223540.

30. Dirix P, Abbeel S, Vanstraelen B, Hermans R, Nuyts S. Dysphagia after chemoradiotherapy for head-and-neck squamous cell carcinoma: dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys. 2009; 10. 75(2):385–392. PMID: 19553033.

31. Eisbruch A, Schwartz M, Rasch C, Vineberg K, Damen E, Van As CJ, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004; 12. 60(5):1425–1439. PMID: 15590174.

32. Gaziano JE. Evaluation and management of oropharyngeal Dysphagia in head and neck cancer. Cancer Control. 2002; Sep-Oct. 9(5):400–409. PMID: 12410179.

33. Miller RM, Groher ME. Speech-language pathology and dysphagia: a brief historical perspective. Dysphagia. 1993; 8(3):180–184. PMID: 8359037.

34. Sessions DG, Zill R, Schwartz SL. Deglutition after conservation surgery for cancer of the larynx and hypopharynx. Otolaryngol Head Neck Surg. 1979; Nov-Dec. 87(6):779–796. PMID: 119201.

35. Mortensen HR, Overgaard J, Specht L, Overgaard M, Johansen J, Evensen JF, et al. Prevalence and peak incidence of acute and late normal tissue morbidity in the DAHANCA 6&7 randomised trial with accelerated radiotherapy for head and neck cancer. Radiother Oncol. 2012; 4. 103(1):69–75. PMID: 22398313.
36. Levendag PC, Teguh DN, Voet P, van der Est H, Noever I, de Kruijf WJ, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: a dose-effect relationship. Radiother Oncol. 2007; 10. 85(1):64–73. PMID: 17714815.

37. Poulsen MG, Riddle B, Keller J, Porceddu SV, Tripcony L. Predictors of acute grade 4 swallowing toxicity in patients with stages III and IV squamous carcinoma of the head and neck treated with radiotherapy alone. Radiother Oncol. 2008; 5. 87(2):253–259. PMID: 18410976.

38. Brizel DM. Radiotherapy and concurrent chemotherapy for the treatment of locally advanced head and neck squamous cell carcinoma. Semin Radiat Oncol. 1998; 10. 8(4):237–246. PMID: 9873101.

39. Manikantan K, Khode S, Sayed SI, Roe J, Nutting CM, Rhys-Evans P, et al. Dysphagia in head and neck cancer. Cancer Treat Rev. 2009; 12. 35(8):724–732. PMID: 19751966.

40. Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003; 11. 349(22):2091–2098. PMID: 14645636.

41. Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefebvre JL, Greiner RH, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004; 5. 350(19):1945–1952. PMID: 15128894.

42. Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004; 5. 350(19):1937–1944. PMID: 15128893.

43. Bhide SA, Gulliford S, Kazi R, El-Hariry I, Newbold K, Harrington KJ, et al. Correlation between dose to the pharyngeal constrictors and patient quality of life and late dysphagia following chemo-IMRT for head and neck cancer. Radiother Oncol. 2009; 12. 93(3):539–544. PMID: 19883951.

44. Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006; 2. 354(6):567–578. PMID: 16467544.

45. Langerman A, Maccracken E, Kasza K, Haraf DJ, Vokes EE, Stenson KM. Aspiration in chemoradiated patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2007; 12. 133(12):1289–1295. PMID: 18086974.

46. Nguyen NP, North D, Smith HJ, Dutta S, Alfieri A, Karlsson U, et al. Safety and effectiveness of prophylactic gastrostomy tubes for head and neck cancer patients undergoing chemoradiation. Surg Oncol. 2006; 12. 15(4):199–203. PMID: 17280829.

47. Stenson KM, MacCracken E, List M, Haraf DJ, Brockstein B, Weichselbaum R, et al. Swallowing function in patients with head and neck cancer prior to treatment. Arch Otolaryngol Head Neck Surg. 2000; 3. 126(3):371–377. PMID: 10722011.

48. Starmer H, Gourin C, Lua LL, Burkhead L. Pretreatment swallowing assessment in head and neck cancer patients. Laryngoscope. 2011; 6. 121(6):1208–1211. PMID: 21484812.

49. Agarwal J, Palwe V, Dutta D, Gupta T, Laskar SG, Budrukkar A, et al. Objective assessment of swallowing function after definitive concurrent (chemo)radiotherapy in patients with head and neck cancer. Dysphagia. 2011; 12. 26(4):399–406. PMID: 21344191.

50. Wu CH, Hsiao TY, Ko JY, Hsu MM. Dysphagia after radiotherapy: endoscopic examination of swallowing in patients with nasopharyngeal carcinoma. Ann Otol Rhinol Laryngol. 2000; 3. 109(3):320–325. PMID: 10737318.

51. Hughes PJ, Scott PM, Kew J, Cheung DM, Leung SF, Ahuja AT, et al. Dysphagia in treated nasopharyngeal cancer. Head Neck. 2000; 7. 22(4):393–397. PMID: 10862024.

52. Nguyen NP, Moltz CC, Frank C, Vos P, Smith HJ, Karlsson U, et al. Dysphagia following chemoradiation for locally advanced head and neck cancer. Ann Oncol. 2004; 3. 15(3):383–388. PMID: 14998839.

53. Corry J, Poon W, McPhee N, Milner AD, Cruickshank D, Porceddu SV, et al. Prospective study of percutaneous endoscopic gastrostomy tubes versus nasogastric tubes for enteral feeding in patients with head and neck cancer undergoing (chemo)radiation. Head Neck. 2009; 7. 31(7):867–876. PMID: 19296528.

54. Bozzetti F. Nutritional support of the oncology patient. Crit Rev Oncol Hematol. 2013; 8. 87(2):172–200. PMID: 23746998.

55. Kiss NK, Krishnasamy M, Loeliger J, Granados A, Dutu G, Corry J. A dietitian-led clinic for patients receiving (chemo)radiotherapy for head and neck cancer. Support Care Cancer. 2012; 9. 20(9):2111–2120. PMID: 22086406.

56. Locher JL, Bonner JA, Carroll WR, Caudell JJ, Keith JN, Kilgore ML, et al. Prophylactic percutaneous endoscopic gastrostomy tube placement in treatment of head and neck cancer: a comprehensive review and call for evidence-based medicine. JPEN J Parenter Enteral Nutr. 2011; 5. 35(3):365–374. PMID: 21527598.
57. Langius JA, Bakker S, Rietveld DH, Kruizenga HM, Langendijk JA, Weijs PJ, et al. Critical weight loss is a major prognostic indicator for disease-specific survival in patients with head and neck cancer receiving radiotherapy. Br J Cancer. 2013; 8. 08. [Epub]. http://dx.doi.org/10.1038/bjc.2013.458.

58. Ehrsson YT, Langius-Eklöf A, Bark T, Laurell G. Percutaneous endoscopic gastrostomy (PEG): a long-term follow-up study in head and neck cancer patients. Clin Otolaryngol Allied Sci. 2004; 12. 29(6):740–746. PMID: 15533171.
59. Silander E, Nyman J, Bove M, Johansson L, Larsson S, Hammerlid E. Impact of prophylactic percutaneous endoscopic gastrostomy on malnutrition and quality of life in patients with head and neck cancer: a randomized study. Head Neck. 2012; 1. 34(1):1–9. PMID: 21374756.
60. Rabinovitch R, Grant B, Berkey BA, Raben D, Ang KK, Fu KK, et al. Impact of nutrition support on treatment outcome in patients with locally advanced head and neck squamous cell cancer treated with definitive radiotherapy: a secondary analysis of RTOG trial 90-03. Head Neck. 2006; 4. 28(4):287–296. PMID: 16287132.

61. Murphy BA. Clinical and economic consequences of mucositis induced by chemotherapy and/or radiation therapy. J Support Oncol. 2007; 10. 5(9 Suppl 4):13–21. PMID: 18046994.
62. Nugent B, Lewis S, O'Sullivan JM. Enteral feeding methods for nutritional management in patients with head and neck cancers being treated with radiotherapy and/or chemotherapy. Cochrane Database Syst Rev. 2010; 3. (3):CD007904. PMID: 20238358.

63. Sanguineti G, Sormani MP, Marur S, Gunn GB, Rao N, Cianchetti M, et al. Effect of radiotherapy and chemotherapy on the risk of mucositis during intensity-modulated radiation therapy for oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2012; 5. 83(1):235–242. PMID: 22104358.

64. Mangar S, Slevin N, Mais K, Sykes A. Evaluating predictive factors for determining enteral nutrition in patients receiving radical radiotherapy for head and neck cancer: a retrospective review. Radiother Oncol. 2006; 2. 78(2):152–158. PMID: 16466819.

65. Kulbersh BD, Rosenthal EL, McGrew BM, Duncan RD, McColloch NL, Carroll WR, et al. Pretreatment, preoperative swallowing exercises may improve dysphagia quality of life. Laryngoscope. 2006; 6. 116(6):883–886. PMID: 16735913.

66. Rosenthal DI, Lewin JS, Eisbruch A. Prevention and treatment of dysphagia and aspiration after chemoradiation for head and neck cancer. J Clin Oncol. 2006; 6. 24(17):2636–2643. PMID: 16763277.

67. Chen AY, Frankowski R, Bishop-Leone J, Hebert T, Leyk S, Lewin J, et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg. 2001; 7. 127(7):870–876. PMID: 11448365.
Table 1
Causes of damage: correlations with head and neck cancer treatment or neurologic damage
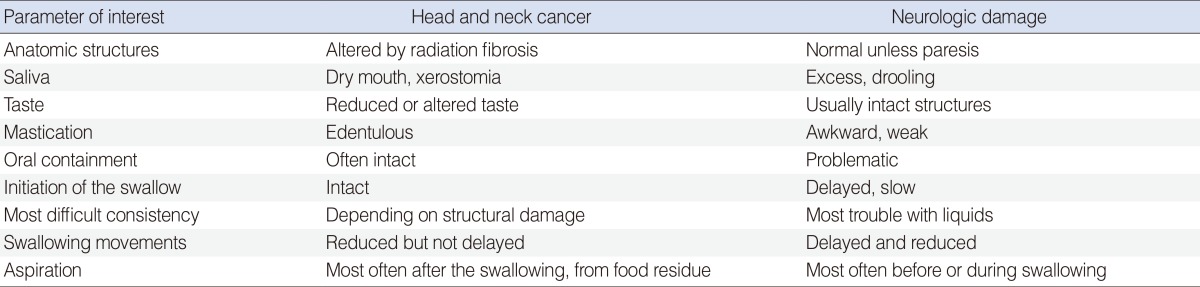
Adapted from Russi et al. [4] with permission from Elsevier.
Table 2
Aspiration in relation of timing of swallowing: pathophysiology
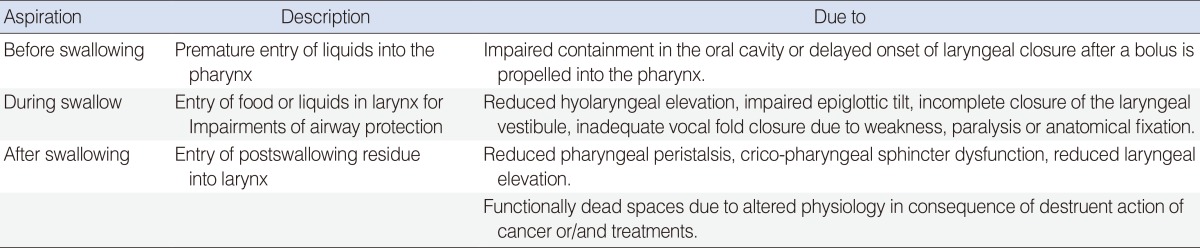
Adapted from Russi et al. [4] with permission from Elsevier.
Table 3
Triggers for dysphagia evaluation

Adapted from Murphy and Gilbert [17] with permission from Elsevier.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download