Abstract
Palatal myoclonus is a rare condition in which there are rhythmic jerky movements of the soft palate and sometimes of the other muscles innervated by the brainstem A particularly annoying symptom is a rhythmic clicking sound in the ear due to the opening and closing of the Eustachian tube. Orofacial buccal dystonia is a focal dystonia with sustained spasms of the masticatory, facial or lingual muscles. The frequent symptoms of this disease have mainly been reported to be involuntary and possibly painful jaw opening, closing, deflecting and retruding, or a combination of the above. However, the subtle and unnoticeable involuntary movement of multiple facial muscles, which might be an infrequent symptom of orofacial buccal dystonia, makes this disease hard to diagnose. Understanding the functional orofacial anatomy that is responsible for the clinical signs and symptoms is necessary for making a proper diagnosis. Here we report on a rare case of palatal myoclonus that was associated with orofacial buccal dystonia, and such a case has not been previously reported. We describe the diagnostic approach and excellent treatment results after Botulinum toxin A (Dysport) injection and proper counseling.
Go to : 
Palatal myoclonus is a movement disorder in which there are rhythmic jerky movements of the soft palate. It is generally classified as somatosound or objective tinnitus and is rare compared to sensorineural tinnitus or subjective tinnitus. According to etiologic factors, platal myoclonus has been classified as two distinct forms: 1) symptomatic palatal myoclonus, which a condition that is secondary to identifiable brainstem or cerebellar disease, and 2) essential palatal myoclonus, which presents clicking tinnitus in the absence of a brain lesion (1). Patients with symptomatic palatal myoclonus show pathologic hypertrophic degeneration of the inferior olive and dentate nucleus, which initiates rhythmic, spontaneous, synchronized discharges within the inferior olive that results in a brainstem "oscillator." In contrast to the known pathologic mechanism of symptomatic palatal myoclonus, there are no reports of the neurophysiology of essential palatal myoclonus. The pathophysiologic hypothesis of this condition is a spontaneous oscillator in other parts of the brainstem, which is related with the palate motor function (nucleus ambiguous-the levator palatini, facial nucleus-the median and paramedian muscles, and the hypoglossal nucleus-the palatopharyngeus and palatoglossus) (1).
Orofacial buccal dystonia is a focal dystonia with sustained spasms of the masticatory, facial or lingual muscles (2). A well-known condition for orofacial buccal dystonia is Meige's syndrome, which is a form of cranial dystonia that's characterized by bilateral dystonic spasms of the facial muscles and frequently by bilateral dystonic spasms of other cranial muscles (2). In this condition, blepharospasm is a most common and disabling manifestation, and it is typically associated with lower facial or oromandibular dystonia. Spasms of the neck and limb muscles may accompany the cranial dystonia and in most cases the cause of spasm is unknown (idiopathic or primary). Secondary Meige's syndrome is encountered in patients with focal brain or brainstem lesions (3).
We recently experienced a rare case of orofacial buccal dystonia in a patient who presented to our hospital's otolaryngology department due to an annoying clicking tinnitus of the ear, and this patient was finally diagnosed as essential palatal myoclonus. To the best of our knowledge, this is the first case report of orofacial buccal dystonia, which predominantly presents as palatal myoclonus. Botulinum toxin A injection and counseling with psychiatric consultation relieved the disabling symptoms of the ear and the orofacial area.
Go to : 
A 22-year-old female presented to the ear, nose and throat (ENT) department with an 18-month history of persistent clicking tinnitus and left facial pain. The patient's tinnitus and sensation of involuntary palatal movement developed about one or two months after starting the use of a wire type orthodontic appliance. There was accompanying involuntary tremor-like dystonia on the left side of her face and buccal area, which was unnoticeable to other people. Clicking tinnitus and involuntary muscle contraction of the face and buccal area prompted the patient to visit several dental, ENT and neurologic clinics; a diagnosis had still not been made after more than a year, and this caused the patient stress.
The tinnitus and dystonic spasm with painful sensation on the left facial area were aggravated over time. Stressful events in her everyday life made the symptoms worse. The patient felt being rejected, sad, frustrated, depressed and even anxious because the painful symptoms remained undiagnosed for a long time. The patient's attempts at relief included herbal medicine, acupuncture and massage, but none of these treatments were successful. The patient also experienced symptom-related depression, anxiety and insomnia, which also created much anxiety among her family members. The patient visited a local neurological clinic where she received brain magnetic resonance imaging (MRI) and an electromygraphic study of her face. Clonazapam was prescribed without a proper diagnosis by one neurologist, but the symptoms were not relieved. The patient identified that the tinnitus was exacerbated by touching the tongue to the palate.
More than 18 months had passed without any definitive diagnosis when the patient first presented to our department. After a careful and long history taking under the suspicion of palatal myoclonus with orofacial buccal dystonia, we thoroughly examined her palate and face. When the patient touched the palate with the tongue, clicking tinnitus was clearly audible and this could be heard by people next to her as one or two rapid clicks per second. Symmetric bilateral contraction of the anterior margin of the soft palate that was synchronous with the patient's clicking tinnitus was observed on both the oral and nasopharyngosocopic examinations. Facial examination revealed weak blepharospasm on the left side of her eyelid. However, we could not detect any noticeable contractions on the buccal area, which is where the patient felt the most severe dystonic movement and pain. We again used a fiberoptic laryngoscope to detect the movement of the buccal muscle and finally found the subtle contraction of the muscles in the left upper lip and buccal mucosal area. The patient also felt an involuntary movement of the muscles around the left nose and chin and even in the deep part of the left anterior neck, but there were no definite tremor-like motions evident on physical examination. The pure-tone, speech and impedance audiometry, including assessment of the static compliance, were normal. Brain MRI did not show any definite abnormal brain findings or brainstem lesion. Consultation with the neurologic department ruled out other neurologic disorders and this was confirmed by the absence of any other accompanying neurologic deficits. All of this led us to diagnose the symptoms as essential palatal myoclonus associated with orofacial buccal dystonia and this was possibly triggered by the orthodontic appliance. The patient refused medication for a long time as she had already unsuccessfully tried different types of muscle relaxants, 5-HT1 agonist, a Levo-dopa agent and anticonvulsants, which were all prescribed by another neurologist.
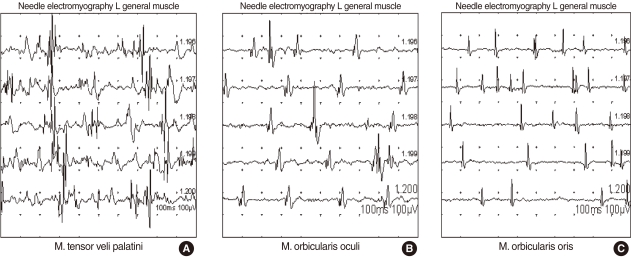
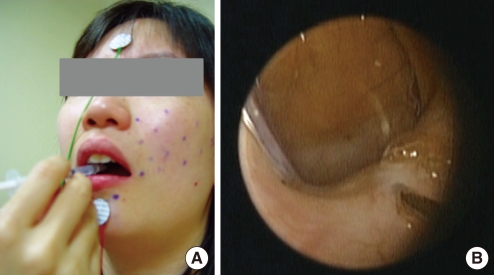
Following an ineffective 1-month course of medical therapy with a combination of muscle relaxant (Baclofen®, Pacific Pharma, Seoul, Korea), anticonvulsant (Rivotril®, Rhoche Korea, Seoul, Korea) and anxiolytic agent (Xanapam®, Myung In Pharm, Hwasung, Korea), the patient was treated with an injection of botulinum toxin A (Dysport®, Ipsen Korea, Seoul, Korea) under the guidance of electromyography (EMG). EMG of the tensor veli palatine, orbicularis oris and orbicularis oculi muscles showed bursts of abnormal electrical activity (Fig. 1). Botulinum toxin A was injected into both sides of the tensor veli palatini muscles bilaterally via the mouth (15 U each) and by a nasal route (10 U each) (Fig. 2). The two most painful and dystonic movement sites on the patient's face (the orbicularis oculi and oris) were selected first and EMG-guided botulinum toxin injections were performed (10-15 U).
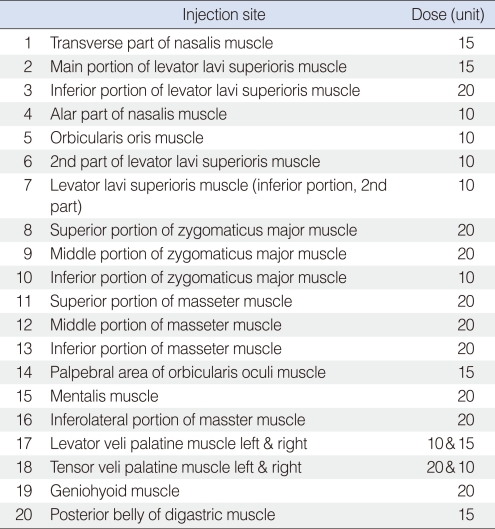
Two days later, she felt decreased clicking tinnitus and a decreased painful sensation with dystonia on her left face. Therefore, botulinum toxin A was injected into every point of the abnormal muscle contraction on the face where the patient pointed and this was confirmed by EMG to have been done. The injected muscle area and the dose of botulinum toxin A are demonstrated in Table 1. On the review at week after injection of botulinum toxin A, her tinnitus and muscle dystonia were markedly decreased. She developed a hypernasal voice and mild facial weakness, but she was satisfied with the treatment. Indeed, the patient wanted to be treated again to have complete cession of her tinnitus and palatal muscle contraction. So, two more consecutive injections into the palatal muscles (30 U in total to each side of the tensor veli palatine muscle) and one more injection into the left buccal area (15 U) were done. At the same time, the patient received counseling, which included general information about brain reorganization and behavioral modification designed to avoid triggering tinnitus and to reduce focusing on the tinnitus and facial muscle dystonia as well. Psychiatric consultation proved beneficial in easing the patient's feelings of depression and anxiety.
At the 2-month follow-up, the palatal myoclonus tinnitus had completely stopped and this was confirmed by nasopharyngoscopic examination. The orofacial buccal dystonia and pain had decreased and what remained was tolerable. Her hypernasal voice and velopharyngeal insufficiency had completely disappeared. Her symptoms did not reappear over the next 4 months. Nonetheless, additional botulinum toxin injections are planned if her symptoms recur.
Go to : 
Palatal myoclonus is known to be a cause of objective tinnitus and is classified as symptomatic if the dysfunction or symptoms are caused by a lesion of the connections between the dentate nucleus, red nucleus and inferior olivary nuclei (Guillain-Mollaret triangle) and as essential if there is no identifiable brain lesion. (1). Contractions of the palatal muscles, including the levator veli palatini muscle and tensor veli palatini muscle cause rapid opening and closing of the Eustachian tube, which produces an objective clicking tinnitus that is clearly audible by the examiner. Palatal myoclonus should be distinguished from middle ear myoclonus, which is another form of objective tinnitus. In middle ear myoclonus, the sound of the tinnitus is a buzzing instead of clicking, and movement of the tympanic membrane rather than palatal tremor is evident. Treatment for middle ear myoclonus involves sectioning of the stapedial and tensor tympani muscles (4).
Orofacial dystonia, which is a part of oromandibular dystonia, has been reported by both neurologists and dentists. Making the diagnosis of dystonia and the distinction between the different focal types such as jaw dystonia, orofacial mandibular dystonia, tongue dystonia and cranial dystonia are purely clinical (2, 3, 5). Making the diagnosis is elusive in patients with unrecognized triggers and who present during periods of quiescence. Further, there is no gold standard diagnostic test or biomarker for testing the validity of the diagnosis. The key to establishing the correct diagnosis includes early detection of the presenting complaint, as well as having an understanding of the functional orofacial anatomy that is responsible for the unique clinical signs and symptoms. In our case, more than 18 months passed before establishing the correct diagnosis and this was followed by proper treatment. Only the suspicion of the possible diagnosis along with thorough knowledge about the disease will prevent a delayed diagnosis. Orofacial dystonia affects more women than men with a mean age of symptom onset between 31 and 58 years (6, 7). The etiology is idiopathic in most cases of orofacial dystonia. Other possible etiologies are that the orofacial dystonia is drug-induced or peripheral disease-induced, or it is associated with postanoxia, neurodegenerative disorder or head injury (8, 9). For our case, the use of an orthodontic appliance might have been a possible etiologic factor that triggered the orofacial dystonia and palatal myoclonus because the patient had a clear memory of her symptom onset as related to wearing the orthodontic appliance. Considering the previous reports about "edentoulous dyskinesia" (10, 11), we can suggest that a possible mechanism in our case is that the malaligned teeth and the orthodontic appliance may have caused an impairment of proprioception in the oral cavity leading to brain reorganization and subsequent development of dystonia. We believe that the temporal and anatomical association between these two events might be considered as the cause since our patient showed orofacial dystonia and palatal myoclonus immediately after using the fixed orthodontic appliance. Stress and emotional factors could be other contributing factors that reinforced the dystonia and palatal myoclonus.
The current lack of knowledge of the exact pathophysiology of dystonia and palatal myoclonus has made it difficult to prescribe specific pharmacologic therapy. Systemic pharmacologic therapy consists of a wide variety of medications, including cholinergics, benzodiazepam, antiparkinsonism drugs, anticonvulsants, muscle relaxants, 5-hydroxytryptopha, levedopa and lithium (1, 2, 8, 9). Surgical therapy is another option, which includes Eustachian tube obliteration and ventilation tube placement (12), dissection of the palatal muscles (13) for the palatal myoclonus and detachment of the involved muscles for the orofacial dystonia (5). Another more widely accepted therapeutic strategy for palatal myoclonus and orofacial dystonia is aimed to control parts of the brain using botulinum toxin, which blocks somatic input and output through peripheral blockage of involuntary muscle movement (6, 14, 15). This toxin is a potent neurotoxin that inhibits the calcium-mediated release of acetylcholine into the synaptic junction, resulting in local chemical denervation and loss of neuronal activity in the targeted organ. It has been used for treating many different types of disorders that showed abnormal movement. Blepharospam, oromandiblar dystonia and hemifacial spasm are representative well known indications for botolum toxin injection therapy. Botolum toxin injection became recognized as a potential treatment for palatal myoclonus in the 1990s (6, 15). The main possible side effects of botulinum toxin treatment are related to a neuromuscular inhibition that exceeds the therapeutic goal or it spreads to the muscle groups adjacent to the muscle that is targeted for treatment. For palatal myoclonus, botulium toxin injection into the palatal muscle can lead to symptoms like velopharyngeal insufficiency, a hypernasal voice or dysphagia. To avoid or minimize side effects, the toxin dosage should be as high as necessary, but as low as possible. However, botulinum toxin injection is considered to be a safe and effective first line therapy. Since there is the lack of experience concerning the optimal dosage of botulinum toxin for special cases like ours, we tried to inject less than 30 U of toxin into each of the problematic sites of the orofacial buccal dystonia and palatal muscles at a single trial. To get the proper therapeutic response, we had to repeat the injection in four consecutive sessions. The dosage guideline for botulinum toxin injection for treating orofacial dytonia and palatal myoclonus should be established in the future. We present here a case of palatal myoclonus associated with orofacial buccal dystonia, and this is the first such report in the medical literature. To establish the correct diagnosis for such a case, early detection of the presenting complaint, as well as a thorough understanding of the functional orofacial anatomy that's responsible for the unique clinical signs and symptoms, is necessary. In our case, injection of botulinum toxin into all the involved muscles was effective. We believe that counseling and psychiatric care also played a role in the therapeutic success. Since botulinum toxin has been proven to be safe and effective for many neuromuscular disorders, and this was also seen in our case, it can be considered to be proper treatment for orofacial buccal dystonia and palatal myoclonus.
Go to : 
References
1. Deuschl G, Mischke G, Schenck E, Schulte-Monting J, Lucking CH. Symptomatic and essential rhythmic palatal myoclonus. Brain. 1990; 12. 113(Pt 6):1645–1672. PMID: 2276039.

2. Clark GT, Koyano K, Browne PA. Oral motor disorders in humans. J Calif Dent Assoc. 1993; 1. 21(1):19–30. PMID: 7682605.
3. Tolosa E, Marti MJ. Blepharospasm-oromandibular dystonia syndrome (Meige's syndrome): clinical aspects. Adv Neurol. 1988; 49:73–84. PMID: 3278555.
4. Badia L, Parikh A, Brookes GB. Management of middle ear myoclonus. J Laryngol Otol. 1994; 5. 108(5):380–382. PMID: 8035114.

5. Balasubramaniam R, Rasmussen J, Carlson LW, Van Sickels JE, Okeson JP. Oromandibular dystonia revisited: a review and a unique case. J Oral Maxillofac Surg. 2008; 2. 66(2):379–386. PMID: 18201628.

6. Tan EK, Jankovic J. Botulinum toxin A in patients with oromandibular dystonia: long-term follow-up. Neurology. 1999; 12. 10. 53(9):2102–2107. PMID: 10599789.

7. Bakke M, Werdelin LM, Dalager T, Fuglsang-Frederiksen A, Prytz S, Moller E. Reduced jaw opening from paradoxical activity of mandibular elevator muscles treated with botulinum toxin. Eur J Neurol. 2003; 11. 10(6):695–699. PMID: 14641515.

8. Jankovic J, Fahn S. Jankovic J, Tolosa E, editors. Dystonic syndromes. Parkinson's disease and movement disorders. 1993. 2nd ed. Baltimore (MD): Williams & Wilkins;p. 337–374.
9. Sankhla C, Lai EC, Jankovic J. Peripherally induced oromandibular dystonia. J Neurol Neurosurg Psychiatry. 1998; 11. 65(5):722–728. PMID: 9810945.

10. Sutcher HD, Underwood RB, Beatty RA, Sugar O. Orofacial dyskinesia: a dental dimension. JAMA. 1971; 5. 31. 216(9):1459–1463. PMID: 4931312.

12. Kwee HL, Struben WH. Tinnitus and myoclonus. J Laryngol Otol. 1972; 3. 86(3):237–241. PMID: 5014915.

13. Jamieson DR, Mann C, O'Reilly B, Thomas AM. Ear clicks in palatal tremor caused by activity of the levator veli palatini. Neurology. 1996; 4. 46(4):1168–1169. PMID: 8780116.

14. Park SN, Park KH, Lee DH, Yeo SW. A case of palatal myoclonus tinnitus treated with botulinm toxin injection. Korean J Otolaryngol-Head Neck Surg. 2005; 9. 48(9):1177–1180.
15. Krause E, Leunig A, Klopstock T, Gurkov R. Treatment of essential palatal myoclonus in a 10-year-old girl with botulinum neurotoxin. Otol Neurotol. 2006; 8. 27(5):672–675. PMID: 16868515.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download