Abstract
Objectives
The aims of this study were to determine the benefits of short-term empirical proton pump inhibitor (PPI) medication on laryngopharyngeal reflux (LPR) and to determine whether scores on the reflux symptom index (RSI) and the reflux finding score (RFS) could be combined to identify subgroups of patients that will more likely to improve with this medication.
Methods
Fifty-one Korean Otolaryngology Board-certified specialists joined this prospective, multi-center, and open-label observational study. A total of 1,142 adult patients with LPR was enrolled for 12 weeks of rabeprazol medication. According to pre-treatment scores on RSI and RFS, patients were divided into 4 subgroups. RFS and RSI were measured repeatedly with a month interval along the treatment period. Changes of RSI and RFS were analyzed in an overall study cohort as well as in each subgroup.
Results
Approximately 40% (n=455) of enrolled patients were followed up until 12 weeks of PPI treatment. Significant improvement in RSI was obtained in 29%, 58%, and 75% of patients after 4, 8, and 12 weeks of PPI medication. RFS was improved in 16%, 42%, and 57% of the patients with 4, 8, and 12 weeks of PPI medication. All subgroups showed improvement regardless of their pre-treatment scores on the RSI and RFS.
Conclusion
Even though RSI and RFS may be used as a general guideline for LPR management, pre-treatment RSI and RFS are not useful in predicting the patients' response to short-term PPI medication in the usual pattern of practice for LPR, which is mostly based on the physical evaluation and history taking.
Larygopharyngeal reflux (LPR) is a retrograde flow of gastric contents into the laryngopharynx, which may result in posterior laryngitis with a constellation of laryngeal symptoms and signs (1). LPR is a commonly encountered problem in otolaryngologic practice. Therefore, it is of significant interest to otolaryngologists (2); LPR is diagnosed in approximately 10% of patients presenting to the outpatient clinic and more than 50% of patients with voice complaints (3).
A diagnosis of LPR is usually based on the response of symptoms to empirical treatment with proton pump inhibitors (PPI). Further investigative modalities, including 24 hour pH monitoring and multi-channel impedance studies are generally reserved for cases of treatment failure (4). However, signs and symptoms of LPR are not specific and can be produced by a wide variety of other conditions, including postnasal drip, infectious agents, and chemical irritants; therefore, its diagnosis may be difficult (5). In addition, laryngeal findings are not always associated with symptom severity (6) and correlation between signs and symptoms of LPR is particularly poor when monitoring therapeutic outcomes (7). As a result, controversy remains regarding how to confirm diagnosis and what comprises appropriate medical management (8).
Belafsky et al. (9, 10) developed two validated assessment instruments in the hope of providing a more consistent and reliable diagnosis of LPR; a nine-item reflux symptom index (RSI) and an eight-item reflux finding score (RFS). Many recent articles have suggested the treatment algorithm or clinical pathway primarily based on these questionnaires; therefore, both indices are believed to be widely used (11). However, there are some controversies regarding their sensitivity, specificity, and correlation between the two instruments, as well as inter-rater or intra-rater reliability in assessment of laryngeal findings (12, 13). According to one of recent nation-wide survey, more than 90% of otolaryngologists do not use these indices during their daily practice (14).
Although H2-receptor antagonists, prokinetic agents, and mucosal cytoprotectants are still used, PPIs are the mainstay of medical treatment (15). A 3-month empirical trial of PPI is generally regarded as a cost-effective approach to initial assessment and management of LPR (16). However, there are some controversies regarding their efficacy as well as the length of the therapeutic trial (17). Although a few trials have analyzed predictors of response to PPI treatment, there are no established predictors of response to PPI therapy (18-21).
The authors conducted a prospective, multi-center, open-label observational study to determine the short-term benefits of rabeprazol (22) medication on LPR. The authors also wanted to know if scores on the RSI and the RFS could be combined to identify subgroups of patients that are more likely to improve with this medication.
A prospective, multi-institutional, and open-label observational study was designed to investigate the effects of rabeprazole short-term treatment in patients with LPR. Fifty-one Korean Otolaryngology Board certified specialists, who were working at 40 different nation-wide secondary or tertiary referral hospitals, participated in this study. Prior to the start of the study, IRB permission was obtained from each institution. A consensus meeting was provided in order to obtain a higher inter- and intra-rater reliability in scoring of RFS. In the meeting, detailed information on the original description of RFS by Belafsky et al. (10) was reiterated to the participating investigators. They were asked to repeatedly rate 8 individual items of RFS while reviewing laryngoscopic pictures or video clips of 7 different representative cases of LPR. An electronic balloting system enabled them to obtain instantaneous feedback by checking the statistics of other investigators' rating scores.
When patients were clinically diagnosed as having LPR, and therfore, PPI medication was determined to be necessary for it, the study protocol was explained to those patients and patients' consents were obtained. Rabeprazol was given to patients without changing each investigator's usual patterns of practice; a total dosage (10 or 20 mg q.d.) per day, administration intervals, and concomitant use of other drugs. Patients were asked to revisit the clinic at 4, 8, and 12 weeks after the first visit. RSI and RFS were obtained at each visit. Collected information regarding the patients' demographic profiles, drug administration, response, and side effects was submitted to a central database server through a web-based electronic-case report form.
Patients who met any of following criteria were excluded from the study; a history of taking PPI within one month, hypersensitivity to any Pariet (Janssen Korea, Hwaseong, Korea) ingredient (rabeprazole sodium) or benzimidazole, moderate to severe hepatic functional impairment, and pregnant or lactating women. From September 2007 to January 2008, a total of 1,142 patients were initially enrolled in the study, and 455 (40%) patients completed the required follow-up visits; they revisited the clinics with an interval of 4 weeks and until more than the 12th week following the initial enrollment in this study.
To see the effects of rabeprazole along the treatment period, 1) values of individual RSI and RFS item or total RSI and RFS scores were compared with those obtained at the previous visit using a mixed model for repeated measurements. 2) Trends of RSI and RFS changes along the treatment period were analyzed using a generalized estimation equation. 3) To minimize inter-rater variability of rating scores, relative RSI and RFS were compared. When post-treatment total RSI or total RFS showed a decrease of 50% or more than initial pre-treatment RSI or RFS, those patients were categorized as responders. A trend of improvement was evaluated by using the number of responders at 4, 8, and 12 weeks.
According to the initial scores of total RSI and RFS, patients were divided into 4 subgroups; RSIhighRFShigh, RSIlowRFShigh, RSIhighRFSlow, and RSIlowRFSlow. RSIhigh was defined as when total RSI is equal to or greater than 14 and RFShigh was defined as when total RFS is equal or greater than 8 (8). The cut-off values of 8 for RFS and 14 for RSI were adopted for the subgrouping of patients according to a recommendation of the previous guideline (8). Repeated measures of ANOVA (SPSS ver. 14.0; SPSS Inc., Chicago, IL, USA) was used to determine which subgroup will obtain more benefit with rabeprazole medication.
At the beginning of the study, 1,142 LPR patients (M:F=470:672) were initially enrolled. Their mean age was 51.9 years old. At 4, 8, and 12 weeks after enrollment, 78% (n=881), 57% (n=661), and 40% (n=455) of the 1,142 enrolled patients revisited outpatient clinics and completed the required evaluations. At 12 weeks, 45%, 39%, 29%, and 39% of RSIhighRFShigh, RSIlowRFShigh, RSIhighRFSlow, and RSIlowRFSlow subgroup populations were followed up, respectively.
Pre-treatment RSI score was 15.13±8.19 (mean±SD), which significantly decreased to 10.77±7.19, 8.00±6.67, and 5.83±6.03 after 4, 8, and 12 weeks of rabeprazole treatment, respectively (Table 1). After 12 weeks of rabeprazole medication, 9 individual items of RSI as well as total RSI showed significant improvement (P<0.0001). There was also a trend toward significant improvement in RSI along the time period (P<0.0001) (Fig. 1).
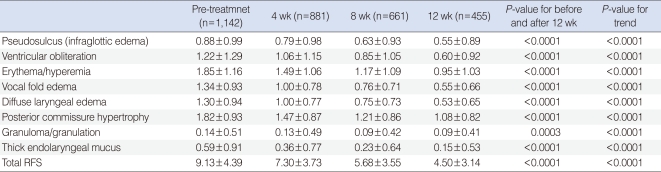
As like the RSI, 8 individual items of RFS as well as total RFS showed significant improvement (P<0.0001) after 12 weeks of rabeprazole medication, when compared with RFS of pre-treatment (Table 2). There was also a trend toward significant improvement in RFS along the time period (P<0.0001) (Fig. 2).
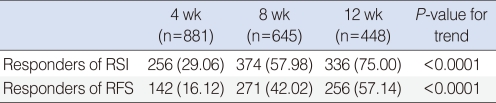
Responders (greater than 50% improvement in RSI or RFS) increased along with the treatment period, and a trend toward improvement was statistically significant (P<0.0001). Responders of RSI and RFS after 12 weeks of treatment reached 75% and 57% in numbers, respectively (Table 3).
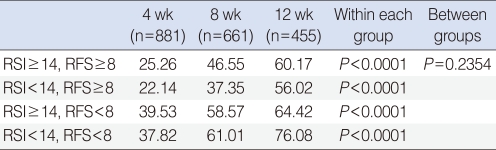
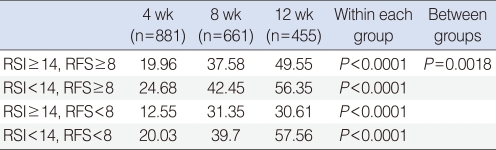
All of the 4 subgroups showed a tendency toward improvement in RSI and RFS along the period of rabeprazole medication (Figs. 1, 2). Within each group, RSI showed significant improvement along the time period (P<0.0001). However, no difference of RSI improvement was observed between the subgroups (P=0.235) (Table 4). As like with RSI, RFS showed significant improvement along the time period (P<0.0001) within each group. In contrast to RSI, a difference of RFS improvement was observed between the subgroups (P=0.002). Further analyses revealed that the responders in RFS were more in the RSI≥14 subgroup (54.00%), when compared with the RSI<14 subgroup (43.04%) with a P-value of 0.012. However, no difference was observed between the RFS ≥ 8 (48.15%) and RFS<8 (50.80%) subgroups (P=0.816) (Table 5).
Treatment of LPR often suffers from poor patient compliance, and therfore, poor symptom improvement (23). In this study, approximately 40% (n=455) of initially enrolled patients were followed up across a period of 12 weeks with PPI treatment. Among those patients, significant RSI and RFS improvement (more than 50%) was observed in 75% and 57% of them, respectively. We could not investigate the reasons why the other 60% of the enrolled patients were lost from follow-up, which is one of the greatest weak points of this study. Even though follow-up rates of each subgroup did not differ significantly from each other (range from 29% to 45%), selection bias might occur between each subgroup, and therefore, it might induce a biased conclusion regarding the response to PPI treatment. In addition, we did not check the patients' compliance regarding medications or behavioral modification, which is another weak point of this study. Since behavioral modification itself may result in marked improvement or complete resolution of patients' discomfort, the compounding effect of it should not be overlooked. According to a study associated with the compliance of LPR patients, only about 50% of patients are taking their medications as prescribed and compliance varies more widely with regard to behavioral modifications (24). Therefore, simplification of the treatment regimen and shortening the period of medical management may increase patient compliance. In this respect, prediction of when and how many LPR patients will improve with PPI empirical treatment may be very important.
In this study, reflux symptoms improved before reflux finding did, which coincides with results of a previous report (6). Significant RSI improvement was obtained in 29%, 58%, and 75% of patients after 4, 8, and 12 weeks of PPI medication. In contrast to RSI, at least 12 weeks of PPI medication was required in order to obtain significant RFS improvement in 57% of patients. Therefore, to assess the effectiveness of PPI treatment in patients with LPR, 2 to 3 months of empirical medication will be needed before confirming favorable changes in reflux symptoms and findings with a probability of higher than 57% to 58%. However, it is worth remembering that approximately 29% of patients may obtain significant improvement in their reflux symptoms even with 4 weeks of PPI medication and that the number of symptom responders will increase along the treatment period of 12 weeks. Moreover, even patients having low-RSI and/or low-RFS may obtain benefit from PPI medications. Patients with contact ulcer or granuloma will be a good example of this; those patients usually have low-RSI (less than 14) and low-RFS (less than 8), however, many of them will improve with PPI medications.
To the best of our knowledge, there are no established predictors of response to PPI medication in patients with LPR. Baseline anxiety levels and heartburn scores were relevant predictors of faster response to PPI (18). Presence of abnormalities in the inter-arytenoid mucosa and true vocal folds were associated with a favorable response to PPI therapy (19). However, the presence of reflux symptoms or reflux findings was not a reliable predictor of good response to PPI (20, 21). In accordance with these previous reports, we could not define reliable symptoms or physical findings for prediction of response to PPI medication. In contrast, positive pharyngeal pH study may be associated with a better symptom response to PPI treatment (20, 21), even though 24 hour pH monitoring and/or multi-channel impedance studies are generally reserved for cases of treatment failure (4).
In our study, all 4 subgroups showed significant improvement in their reflux symptoms and findings with PPI medication, which means that their pre-treatment RSI or RFS values are not useful predictors of the response to short-term PPI medication. This phenomenon may be explained in part by the placebo-effect, not only of the patients but also of the investigaters, since this study was conducted not as a blinded and not as a randomized design. A randomized, double-blinded, placebo-controlled study revealed improvement in RFS and RSI in both placebo and PPI groups after 6 weeks of treatment with PPI, even though improvements were greater in the PPI group in several parameters (17). Subjective criteria for the diagnosis of LPR and no uniform criteria in the enrollment of patient could be another limitation of this study. No standardization in the dose of rabeprazole could be another variables. The effects of concomitant drug usage or co-morbidity were not taken into account while analyzing the response to PPI. Moreover, the effects of total dosage and dosing of PPI as well as the timing of PPI medication regarding to food intakes were also not adjusted before analyzing the data, which could significantly influence on the outcome of drug response, therefore, on the conclusion of this study. Unproven inter-rater and/or intra-rater reliability for assessment of RFS might also be one of the reasons, even though the investigators were educated through a consensus meeting in order to reach a greater inter-rater reliability in scoring RFS.
Despite many constraints of this study, this is one of the few reports involving relatively large numbers of LPR patient (n=1,142) as well as broad spectrum of physicians (n=40) as co-investigators with a prospective design, which probably can override the above mentioned weaknesses. In summary, the authors suggest that pre-treatment RSI and RFS are not reliable predictors for the outcome of PPI treatment in the usual pattern of practice provided for LPR patients, which is largely based on the physical evaluation and history taking.
References
1. Koufman JA. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): a clinical investigation of 225 patients using ambulatory 24-hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope. 1991; 4. 101(4 Pt 2 Suppl 53):1–78. PMID: 1895864.
2. Book DT, Rhee JS, Toohill RJ, Smith TL. Perspectives in laryngopharyngeal reflux: an international survey. Laryngoscope. 2002; 8. 112(8 Pt 1):1399–1406. PMID: 12172252.

3. Fraser AG. Review article: gastro-oesophageal reflux and laryngeal symptoms. Aliment Pharmacol Ther. 1994; 6. 8(3):265–272. PMID: 7918920.

4. Bove MJ, Rosen C. Diagnosis and management of laryngopharyngeal reflux disease. Curr Opin Otolaryngol Head Neck Surg. 2006; 6. 14(3):116–123. PMID: 16728885.
5. Tutuian R, Castell DO. Diagnosis of laryngopharyngeal reflux. Curr Opin Otolaryngol Head Neck Surg. 2004; 6. 12(3):174–179. PMID: 15167025.
6. Belafsky PC, Postma GN, Koufman JA. Laryngopharyngeal reflux symptoms improve before changes in physical findings. Laryngoscope. 2001; 6. 111(6):979–981. PMID: 11404607.

7. Qadeer MA, Swoger J, Milstein C, Hicks DM, Ponsky J, Richter JE, et al. Correlation between symptoms and laryngeal signs in laryngopharyngeal reflux. Laryngoscope. 2005; 11. 115(11):1947–1952. PMID: 16319603.

8. Ford CN. Evaluation and management of laryngopharyngeal reflux. JAMA. 2005; 9. 28. 294(12):1534–1540. PMID: 16189367.

9. Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the reflux symptom index (RSI). J Voice. 2002; 6. 16(2):274–277. PMID: 12150380.

10. Belafsky PC, Postma GN, Koufman JA. The validity and reliability of the reflux finding score (RFS). Laryngoscope. 2001; 8. 111(8):1313–1317. PMID: 11568561.

11. Mesallam TA, Stemple JC, Sobeih TM, Elluru RG. Reflux symptom index versus reflux finding score. Ann Otol Rhinol Laryngol. 2007; 6. 116(6):436–440. PMID: 17672246.

12. Kelchner LN, Horne J, Lee L, Klaben B, Stemple JC, Adam S, et al. Reliability of speech-language pathologist and otolaryngologist ratings of laryngeal signs of reflux in an asymptomatic population using the reflux finding score. J Voice. 2007; 1. 21(1):92–100. PMID: 16546351.

13. Branski RC, Bhattacharyya N, Shapiro J. The reliability of the assessment of endoscopic laryngeal findings associated with laryngopharyngeal reflux disease. Laryngoscope. 2002; 6. 112(6):1019–1024. PMID: 12160267.

14. Karkos PD, Benton J, Leong SC, Karkanevatos A, Badran K, Srinivasan VR, et al. Trends in laryngopharyngeal reflux: a British ENT survey. Eur Arch Otorhinolaryngol. 2007; 5. 264(5):513–517. PMID: 17404773.

15. Gupta R, Sataloff RT. Laryngopharyngeal reflux: current concepts and questions. Curr Opin Otolaryngol Head Neck Surg. 2009; 6. 17(3):143–148. PMID: 19395970.

16. Fass R. Empirical trials in treatment of gastroesophageal reflux disease. Dig Dis. 2000; 18(1):20–26. PMID: 10729734.

17. Reichel O, Dressel H, Wiederanders K, Issing WJ. Double-blind, placebo-controlled trial with esomeprazole for symptoms and signs associated with laryngopharyngeal reflux. Otolaryngol Head Neck Surg. 2008; 9. 139(3):414–420. PMID: 18722223.

18. Siupsinskiene N, Adamonis K, Toohill RJ, Sereika R. Predictors of response to short-term proton pump inhibitor treatment in laryngopharyngeal reflux patients. J Laryngol Otol. 2008; 11. 122(11):1206–1212. PMID: 18331659.

19. Park W, Hicks DM, Khandwala F, Richter JE, Abelson TI, Milstein C, et al. Laryngopharyngeal reflux: prospective cohort study evaluating optimal dose of proton-pump inhibitor therapy and pretherapy predictors of response. Laryngoscope. 2005; 7. 115(7):1230–1238. PMID: 15995512.

20. Williams RB, Szczesniak MM, Maclean JC, Brake HM, Cole IE, Cook IJ. Predictors of outcome in an open label, therapeutic trial of high-dose omeprazole in laryngitis. Am J Gastroenterol. 2004; 5. 99(5):777–785. PMID: 15128336.

21. Fraser AG, Morton RP, Gillibrand J. Presumed laryngo-pharyngeal reflux: investigate or treat? J Laryngol Otol. 2000; 6. 114(6):441–447. PMID: 10962677.

22. Lam PK, Ng ML, Cheung TK, Wong BY, Tan VP, Fong DY, et al. Rabeprazole is effective in treating laryngopharyngeal reflux in a randomized placebo-controlled trial. Clin Gastroenterol Hepatol. 2010; 9. 8(9):770–776. PMID: 20303417.

23. Friedman M, Maley A, Kelley K, Pulver T, Foster M, Fisher M, et al. Impact of pH monitoring on laryngopharyngeal reflux treatment: improved compliance and symptom resolution. Otolaryngol Head Neck Surg. 2011; 4. 144(4):558–562. PMID: 21493235.
24. Giacchi RJ, Sullivan D, Rothstein SG. Compliance with anti-reflux therapy in patients with otolaryngologic manifestations of gastroesophageal reflux disease. Laryngoscope. 2000; 1. 110(1):19–22. PMID: 10646709.

Fig. 1
Changes of reflux symptom index (RSI) in each subgroup with short-term rabeprazole medication. According to initial scores of total RSI and reflux finding score (RFS), patients were divided into 4 subgroups. Changes of score on RSI were plotted along the 12 weeks period of rabeprazole medication in both a total patient cohort and each subgroup. All of the 4 subgroups as well as the total patient cohort showed a tendency toward improvement in RSI along the treatment period (P<0.0001).
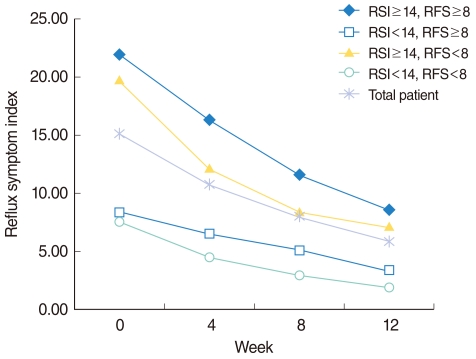
Fig. 2
Changes of reflux finding scores (RFS) in each subgroup with short-term rabeprazole medication. According to initial scores of total reflux symptom index (RSI) and RFS, patients were divided into 4 subgroups. Changes of score on RFS were plotted along the 12 weeks period of rabeprazole medication in both a total patient cohort and each subgroup. All of the 4 subgroups as well as the total patient cohort showed a tendency toward improvement in RFS along the treatment period (P<0.0001).
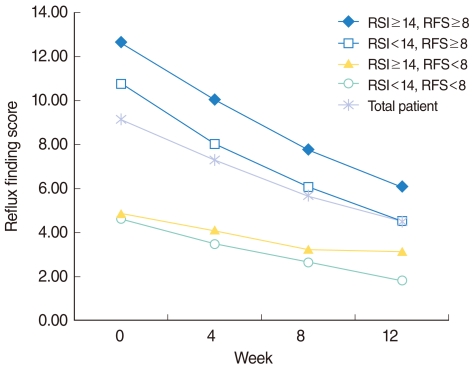
Table 1
Changes of reflux symptom index (RSI) before and after 4, 8, and 12 weeks of rabeprazole medication
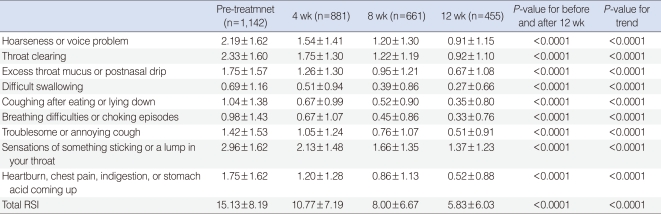
Table 2
Changes of reflux finding scores (RFS) before and after 4, 8, and 12 weeks of rabeprazole medication




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download