Abstract
Objectives
The primary aim of this study was to assess whether one can use levels of nasal nitric oxide (nNO) and exhaled nitric oxide (eNO) as a means of evaluation in allergic rhinitis.
Methods
We used a chemiluminescence analyzer to measure nNO and eNO in normal controls (n=34) and allergic rhinitis patients (n=35), and compared these measurements with various parameters of clinical symptoms and laboratory data.
Results
Mean nNO (389±119 ppb) in allergic rhinitis patients was significantly higher than normal controls (276±88 ppb). Without asthma, mean eNO (64.8±55.9 ppb) in allergic rhinitis patients was significantly higher than normal controls (33.0±24.0 ppb). In the persistent allergic rhinitis group, eNO concentration was significantly higher, while nNO concentration was significantly lower than the intermittent group.
Exhaled nitric oxide (eNO) is NO in the lower airway measured by oral exhalation. Many workers have investigated eNO as a useful non-invasive biomarker of asthma, which is correlated with eosinophilic airway inflammation. In the past, patients had to go to testing laboratories and undergo eNO measurement with complicated equipment, but portable analyzers have now been developed and are widely used in asthma clinics.
Nasal nitric oxide (nNO) refers to NO measured in air aspirated from the nasal cavity and produced continuously in the paranasal sinuses without inflammatory stimuli. It is toxic to bacteria, viruses, fungi and tumor cells, and may play a role in protecting the upper airway. In response to inflammatory stimuli, nNO production increases due to induction of inducible NO synthase (iNOS) in upper respiratory epithelial cells.
With regard to rhinologic diseases, primary ciliary dyskinesia patients lack iNOS in their epithelial cells and nNO levels are very low. Nasal NO is also low in nasal polyps, chronic rhinosinusitis and cystic fibrosis, due to obstruction of the osteomeatal unit, which inhibits release of the nNO produced in the paranasal sinuses. On the other hand, nNO production in inflammatory diseases such as allergic rhinitis is high because of the elevated iNOS production [1].
In this study we measured nNO and eNO in patients with allergic rhinitis and compared them with a control group, to see whether their levels are correlated with various clinical parameters, and whether measurement of nNO and eNO levels could be useful for evaluating allergic rhinitis.
Thirty-five patients with allergic rhinitis were included in the study. The control group consisted of 34 patients with deviated nasal septa who did not have inflammatory diseases of the nasal cavities.
The control group underwent NO measurement preoperatively. Allergic rhinitis was ruled out by either multiple antigen simultaneous tests (MASTs) or allergic skin tests. Sinusitis was ruled out by paranasal sinus plain X-ray or computed tomography. The mean age of control group was 26.9±11.0 years and consisted of 17 males and 17 females.
The allergic rhinitis group was diagnosed with allergic rhinitis by history-taking and MAST or skin tests. They did not display any symptoms or X-ray findings of rhinosinusitis. The group had a mean age of 22.7±8.7 years and consisted of 25 males and 10 females.
We excluded pediatric patients below 15, patients who might have had asthma based on pulmonary function tests, patients who had undergone nasal operations, and patients who had taken medication for nasal symptoms within the previous week.
NO concentration was measured with a chemiluminescence analyzer (Sievers nitric oxide analyzer, NOA 280i; GE Analytical Instruments, Boulder, CO, USA), according to American Thoracic Society/European Respiratory Society (ATS/ERS) recommendations [2].
To isolate the nasal cavity from the lower airway, velopharyngeal closure was achieved by inhalation to total lung capacity followed by oral exhalation maintaining an expiratory pressure of more than 10 cm H2O. Air was then aspirated at 700 mL/minute with a suction pump, and nNO samples were taken at 200 mL/minute in the midstream of the aspirated air flow, and analyzed with a computer data acquisition program. A latex nasal olive was inserted in one nostril, making a tight seal, while the contralateral nostril was left open. The plateau from the computer data system was read as the measured value. All measured values were determined by averaging two samples with less than a 10% difference in readings.
Exhaled NO, like nNO, was measured during velopharyngeal closure, and the plateau from the computer was chosen when the expiratory flow rate reached 50 mL/second. Values were averages of two samples differing by less than 10%.
As ambient NO concentrations can influence nNO, not like eNO, nNO was corrected by subtracting ambient NO from the measured NO. Hence nNO in this study refers to corrected NO. In the controls with nasal septal deviations, NO measurements were obtained twice in both nasal cavities because of possible differences between the nasal cavities.
All participants underwent a MAST or allergic skin test, and measurements of total IgE level and eosinophil count, together with a peripheral blood test and an olfactory function test using the Korean version of Sniffin' sticks (KVSS) threshold test. A questionnaire enquired about smoking history, personal and family history of allergic disorders including asthma, atopy, and allergic rhinitis, history of otolaryngologic surgery, medication, symptoms of allergic rhinitis such as nasal obstruction, watery rhinorrhea, and itching, and symptoms of rhinosinusitis and asthma. The questionnaire also included a symptom severity score and a duration score based on Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines and the symptom scores. Every symptom score was measured using a visual analogue scale from none (score 0) to very severe (score 5). The study was approved by the Hanyang University Guri Hospital Institutional Review Board.
For statistical analysis we used SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). Mean differences between groups were analyzed using Student's t-test, the chi-square test, Fisher's exact test, the Mann-Whitney U-test, and the Kruskal-Wallis test. Pearson & Spearman's correlation test was used to assess associations between variables, and univariate and multivarate logistic regression analysis was used for dependant variables. P<0.05 were considered statistically significant.
There were no statistically significant differences in gender, age, height, weight, and BMI, between the control group and the allergic rhinitis patient group.
Mean nNO concentration in the control group was 276.4±88.1 ppb and eNO concentration, 33.0±21.0 ppb. Mean nNO concentration in the allergic rhinitis group was 388.0±119.2 ppb, significantly higher than that of the normal control group (P<0.0001). Mean eNO concentration was 64.8±55.9 ppb, also significantly higher than that of the normal control group (P=0.003) (Fig. 1).
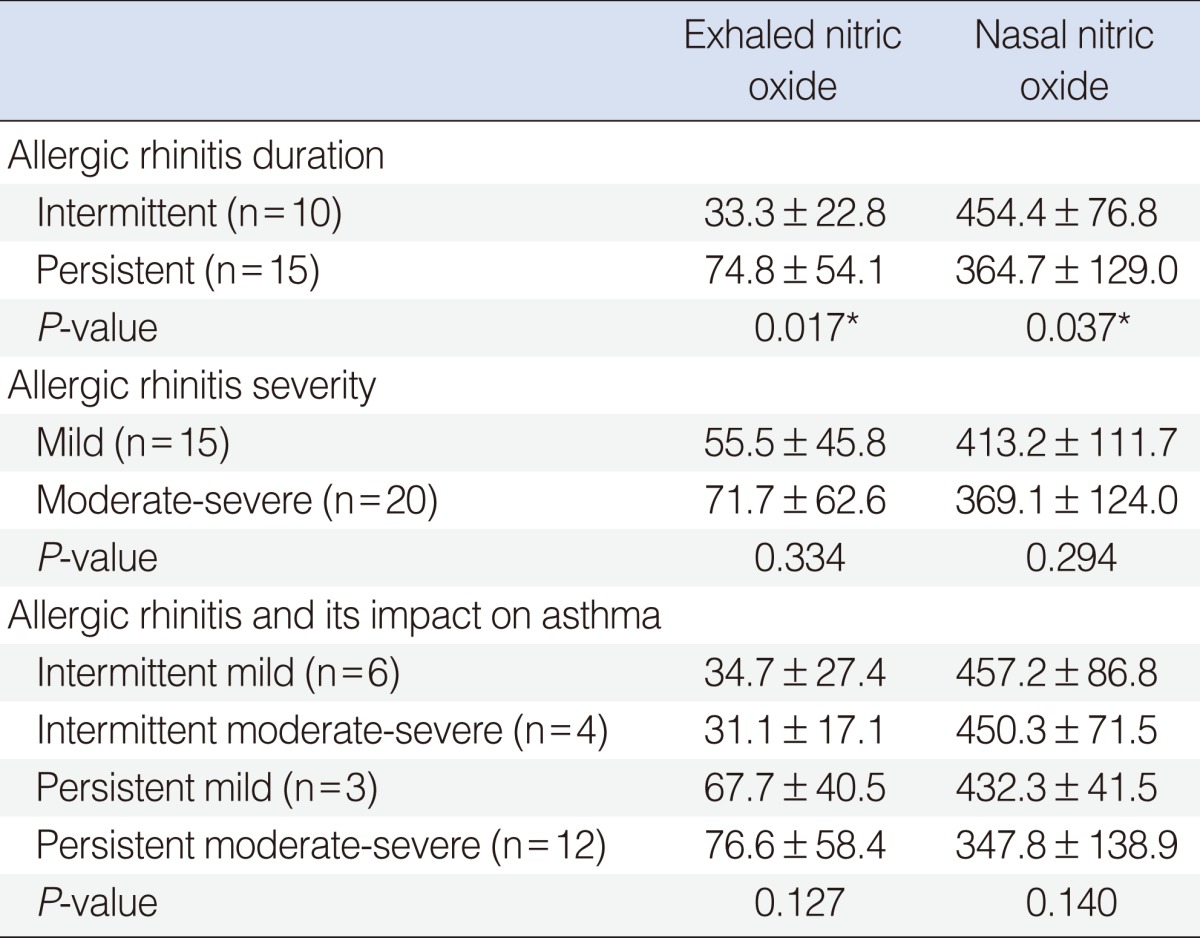
In the persistent group, eNO concentration was significantly higher, while nNO concentration was significantly lower than the intermittent group. In the severe group, eNO concentration was higher, and nNO concentration lower than mild to moderate group, although the differences were not statistically significant. Levels of nNO and eNO were similar as 4 classifications of ARIA guidelines (Table 1).
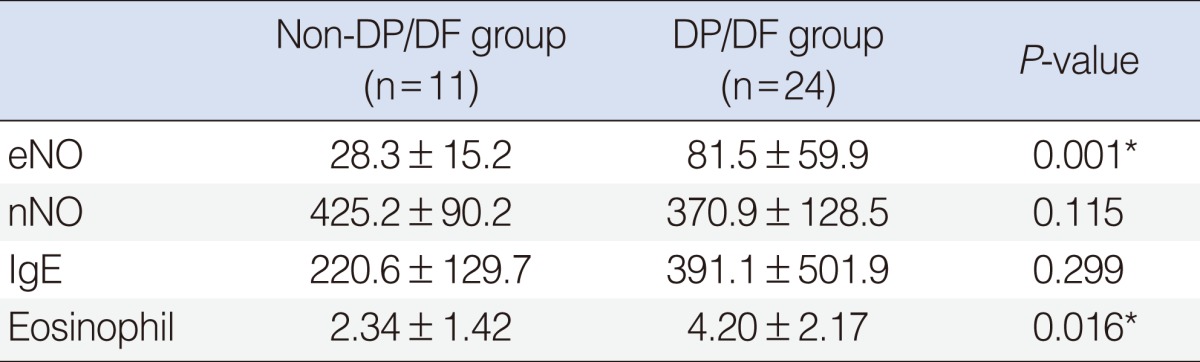
Among the allergic rhinitis patients, eNO and eosinophil count were significantly higher in the Dermatophagoides pteronyssinus/Dermatophagoides farinae (DP/DF) positive group than non-DP/DF positive group (other allergen positive group), but nNO and IgE weren't (Table 2).
There were statistically significant correlations between eNO and the symptom scores on watery rhinorrhea and sneezing, and between eNO and the sum of allergic symptom scores (Fig. 2). There was a weak correlation between eNO and eosinophil count, although not statistically significant. There was no statistically significant correlation between eNO and total IgE level in the allergic rhinitis patient group.
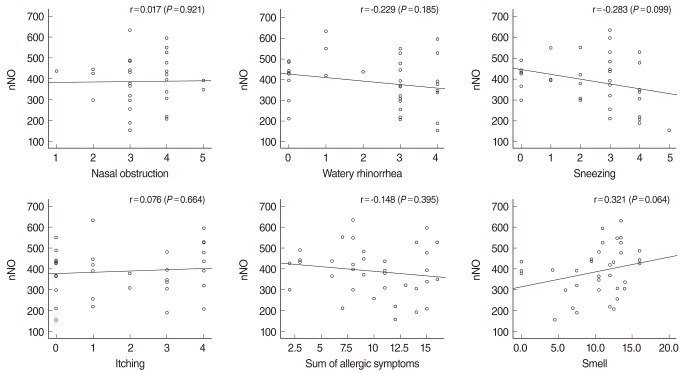
There was no statistically significant correlation between nNO and total IgE level, or between nNO and eosinophil count. There were no statistically significant correlations between nNO and scores on allergic symptoms (Fig. 3). There was no statistically significant correlation between nNO and eNO in either the control group or the allergic rhinitis group.
In this study, we found that the nNO concentration in the allergic rhinitis patients was significantly higher than in the normal controls. Kawamoto et al. [3,4] provide indirect support for this finding. Those investigations measured not nNO itself but the enzyme, NOS which catalyzes the synthesis of NO from L-arginine in the nasal epithelial cells. They reported that expression of iNOS was elevated in the nasal epithelial cells of allergic rhinitis patients, especially in the nasal submucosal glands. This can be explained by assuming that the continuous mucosal inflammation in allergic rhinitis increases iNOS activity. Recently, nNO measurement has become relatively standardized by the ATS/ERS guidelines, and many other studies have measured nNO itself [2]. Our study was also conducted according to these guidelines, whose essential points are a constant aspiration flow rate (700 mL/minute), and correction for ambient NO.
Most of studies about nNO in allergic rhinitis have reported that nNO increases in allergic rhinitis. Kharitonov et al. [5] compared nNO levels in subjects with symptomatic seasonal allergic rhinitis, some of whom had concomitant asthma, and in normal subjects, measured over 2 years. Regardless of whether patients had concomitant asthma or not, nNO was 1.5 times higher in patients with allergic rhinitis than in normal subjects, and there was no seasonal effect on nNO. Djupesland et al. [6] reported the same result in symptomatic seasonal allergic rhinitis had a higher nNO output than normal subjects. Arnal et al. [7] compared baseline nNO between allergic rhinitis patients and normal controls. They found that, whether the allergy was seasonal or perennial, and the patients had symptoms or not, subjects with allergic rhinitis had higher nNO concentrations than normal subjects; this could reflect permanent inflammation of the nasal cavity and the paranasal sinus mucosa in the allergic rhinitis patients.
On the other hand, several groups reported that allergic rhinitis didn't affect on nNO levels. Maniscalco et al. [8] reported that basal nNO concentrations in allergic rhinitis patients were higher than in controls but not significantly. Palm et al. [9] reported that allergic rhinitis patients had normal nNO levels but elevated eNO levels. They suggests that although nNO production may be upregulated in the nasal mucosa of patients with allergic rhinitis, the effect may be counteracted by swelling of the mucosa and secretions, so impairing diffusion of NO from the paranasal sinuses; in addition the high background levels of NO from constitutive sources in the nose may hide small increases in mucosal NO output [9]. As our results, if symptoms are severe or persist for a very long time, nNO may not increase.
The increased NO in allergic rhinitis has several pathophysiologic consequences, including vasodilation and modification of sensory nerve endings. The increased NO aggravates nasal obstruction, rhinorrhea and sneezing. In the other words, it appears that the higher the NO, the severer the symptoms. However, there was no significant correlation between nNO and the severity of allergic rhinitis symptoms in this study, it is similar with the study of Baraldi et al. [10].
We found that not only nNO but also eNO were significantly higher in allergic rhinitis patients than normal controls. Hence, eNO may be elevated in allergic rhinitis patients that do not have concomitant asthma, which suggests that physicians should see whether patients have allergic rhinitis when they use NO as diagnostic tools of asthma or monitoring tools of treatment efficacy. Scott et al. [11] showed that atopy was significantly associated with higher levels of eNO. They reported that the level of eNO in non-atopic asthmatic participants was no different from that in the non-atopic non-asthmatic group and concluded that eNO behaves as a biomarker of atopy and of the "allergic asthma" phenotype, rather than of asthma itself. In our study, eNO was especially high in the DP/DF-positive group, presumably because they had a strong atopic tendency, but nNO levels showed no correlation with house dust mite reactivity. The patients with severe symptoms of allergic rhinitis tended to have more increased eNO in our study.
Hanazawa et al. [12] showed that eotaxin causes chemotaxis of eosinophils with a clinically symptomatic inflammatory response in the nasal mucosa, and that eosinophil recruitment accompanies a significant increase in nNO. We did not find a correlation between nNO and eosinophil count in peripheral blood, and the relationship between nNO and eosinophil count in nasal mucosa tissue needs to be investigated.
In our study, eNO was higher in moderate severe and persistent symptoms group than mild and intermittent group, whereas nNO was lower. This indicates that eNO reflects the increase in the severity of lower airway inflammation according to increased upper airway inflammation; although nNO production might be expected to increase in patients with severe and persistent allergic symptoms, it actually decreases due to severe mucosal swelling resulting in impaired NO diffusion. Kharitonov et al. [5] showed that in subjects with the severest symptoms of seasonal allergic rhinitis who were challenged with pollen allergen, nNO was lowest 1 hour after challenge, nNO levels returned to their baseline after 4 hours. Our data also demonstrates that nNO production can be decreased with severe and persistent allergic symptoms and we think it is because of special output structures, such as inferior turbinate and small sinus ostium, unlike eNO from the lower respiratory tract which doesn't have those structures.
In conclusion, nNO measurements can be used to predict allergic rhinitis in patients who are not able to undergo allergic tests or invasive procedures. However, they should be interpreted with caution in patients with severe or persistent symptoms, because nNO levels may be low rather than high. eNO has been used to diagnose asthma and monitor treatment efficacy, but since atopy such as allergic rhinitis is associated with higher levels of eNO, such eNO measurements should be made in conjunction with evaluation of atopy. We suggest that the standardized and simplified measurement techniques and established guidelines for standard values will lead to increased usefulness of nNO and eNO measurements in allergic disorders.
References
1. Scadding G. Nitric oxide in the airways. Curr Opin Otolaryngol Head Neck Surg. 2007; 8. 15(4):258–263. PMID: 17620900.

2. American Thoracic Society. European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005; 4. 171(8):912–930. PMID: 15817806.
3. Kawamoto H, Takumida M, Takeno S, Watanabe H, Fukushima N, Yajin K. Localization of nitric oxide synthase in human nasal mucosa with nasal allergy. Acta Otolaryngol Suppl. 1998; 539:65–70. PMID: 10095865.
4. Kawamoto H, Takeno S, Yajin K. Increased expression of inducible nitric oxide synthase in nasal epithelial cells in patients with allergic rhinitis. Laryngoscope. 1999; 12. 109(12):2015–2020. PMID: 10591366.

5. Kharitonov SA, Rajakulasingam K, O'Connor B, Durham SR, Barnes PJ. Nasal nitric oxide is increased in patients with asthma and allergic rhinitis and may be modulated by nasal glucocorticoids. J Allergy Clin Immunol. 1997; 1. 99(1 Pt 1):58–64. PMID: 9003212.

6. Djupesland PG, Chatkin JM, Qian W, Cole P, Zamel N, McClean P, et al. Aerodynamic influences on nasal nitric oxide output measurements. Acta Otolaryngol. 1999; 119(4):479–485. PMID: 10445065.
7. Arnal JF, Didier A, Rami J, M'Rini C, Charlet JP, Serrano E, et al. Nasal nitric oxide is increased in allergic rhinitis. Clin Exp Allergy. 1997; 4. 27(4):358–362. PMID: 9146927.

8. Maniscalco M, Sofia M, Carratu L, Higenbottam T. Effect of nitric oxide inhibition on nasal airway resistance after nasal allergen challenge in allergic rhinitis. Eur J Clin Invest. 2001; 5. 31(5):462–466. PMID: 11380599.

9. Palm JP, Alving K, Lundberg JO. Characterization of airway nitric oxide in allergic rhinitis: the effect of intranasal administration of L-NAME. Allergy. 2003; 9. 58(9):885–892. PMID: 12911417.

10. Baraldi E, Azzolin NM, Carra S, Dario C, Marchesini L, Zacchello F. Effect of topical steroids on nasal nitric oxide production in children with perennial allergic rhinitis: a pilot study. Respir Med. 1998; 3. 92(3):558–561. PMID: 9692122.

11. Scott M, Raza A, Karmaus W, Mitchell F, Grundy J, Kurukulaaratchy RJ, et al. Influence of atopy and asthma on exhaled nitric oxide in an unselected birth cohort study. Thorax. 2010; 3. 65(3):258–262. PMID: 20335297.

12. Hanazawa T, Antuni JD, Kharitonov SA, Barnes PJ. Intranasal administration of eotaxin increases nasal eosinophils and nitric oxide in patients with allergic rhinitis. J Allergy Clin Immunol. 2000; 1. 105(1 Pt 1):58–64. PMID: 10629453.

Fig. 1
Nasal & exhaled nitric oxide (NO) in the control and allergic rhinitis groups. Nasal & exhaled NO were significantly higher in allergic rhinitis group than control group.
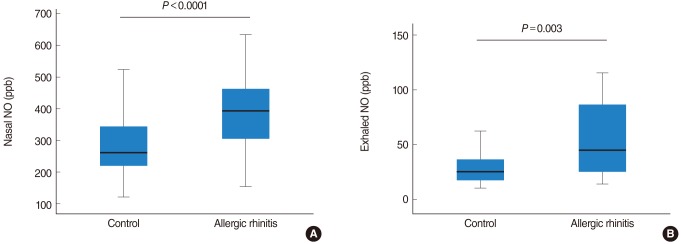
Fig. 2
Correlation between exhaled nitric oxide (eNO) and symptoms in the allergic rhinitis group. There was significant correlation between eNO and watery rhinorrhea, sneezing, sum of symptoms. Other symptoms showed no significant correlation with eNO.
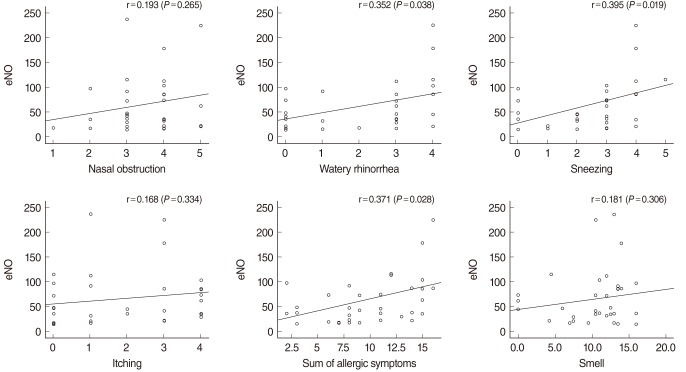
Fig. 3
Correlation between nasal nitric oxide (nNO) and symptoms in the allergic rhinitis group. There was no significant correlation between nNO and symptoms.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download