This article has been
cited by other articles in ScienceCentral.
Abstract
Objectives
Although human papillomavirus (HPV) infection is considered as a favorable prognostic factor in oropharyngeal cancer, the prognosis of HPV-associated tonsil cancer has rarely been studied especially when surgery was the main treatment. In this study, the authors investigated the effect of p16 over-expression (HPV infection) on tonsil cancer prognosis according to the type of treatment, HPV presence by PCR, and expression of p53 and epidermal growth factor receptor (EGFR) by immunohistochemistry (IHC).
Methods
Medical records of 33 tonsil cancer patients were reviewed. Using formalin-fixed and paraffin-embedded tumor specimens, PCR-based genotyping of HPV and IHC of p16, p53 and EGFR were performed. The effects of HPV presence and the expression of IHC markers were analyzed on the recurrence-free survival. Five-year disease-free survival (DFS) rates were evaluated according to p16 expression status.
Results
An over-expression of p16 was observed in 27 (81.9%) out of 33 cases. Surgery-based treatment was provided for 21 (63.6%) patients. There was no association between p16 immunoreactivity and HPV presence, nor with p53 and EGFR expression. Regardless of main treatment modalities, five-year DFS did not differ by p16 expression status (P=0.051). However, over-expression of p16 was associated with a lower recurrence in multivariable analyses (P=0.046).
Conclusion
Regardless of main treatment modalities, an over-expression of p16 (HPV infection) is associated with a lower recurrence in tonsil cancers. However it is not associated with simple HPV presence or p53 and EGFR over-expression.
Go to :

Keywords: Human papillomavirus, Oropharyngeal neoplasms, Therapeutics, Immunohistochemistry, Prognosis, p16 (INK4A)
INTRODUCTION
Certain human papillomaviruses (HPVs) are the most important etiologic factors of a subset of head and neck cancers, including oropharyngeal squamous cell cancers (OPSCCs). Known since 1983 in oral cancers [
1], evidence has consistently implicated persistent HPV infection in malignant transformation of tonsillar epithelium and in uterine cervical cancer [
2]. Recently the prognostic importance of HPV has been demonstrated, showing that HPV-positive patients have better survival than HPV-negative patients [
3-
7].
With the increased molecular evidence for HPVs, biomarkers and their associations with carcinogenesis in OPSCCs have been elaborated. Viral oncoproteins, like E6, E7 deregulates p53 and pRb-related cell cycle control mechanisms, leading to immortalization [
3]. Over-expression of p16 is consistent with a regulatory feedback mechanism to compensate for the E7 induced loss of pRb, often used as a surrogate marker of HPV oncogene expression [
3]. Epidermal growth factor receptor (EGFR) is the tyrosine kinase receptor normally present in epithelial tissues. EGFR is expressed in most of head and neck squamous cell carcinomas (HNSCCs), associated with aggressive behavior and poor prognosis [
8].
It has become increasingly evident that p16 over-epxression is a useful diagnostic tool for HPV testing as well as a prognostic factor predicting the clinical outcome based on high concordance of p16 immunohistochemistry (IHC) staining and HPV-
in situ hybridization (ISH) on OPSCCs [
9,
10].
However, HPV-associated tonsil cancer has rarely been studied, especially when the surgery was a main treatment modality. We investigated the effect of p16 over-expression (HPV infection) on the prognosis of tonsil cancers according to the types of treatment, HPV presence by PCR, and expression status of p53 and EGFR by IHC.
Go to :

MATERIALS AND METHODS
Thirty-three tonsil cancer cases, diagnosed from September 1996 to September 2006, were retrospectively reviewed. Their clinical data and formalin-fixed, paraffin-embedded tumor specimens were collected. Data of smoking history was collected on Samsung Cancer Center Data Bank.
Using formalin-fixed, paraffin-embedded tumor specimens, HPV DNA detection and immunohistochemical staining of p16, p53, and EGFR were performed. Since patients who underwent surgery for tonsil cancer had many specimen blocks, the typical sections that included the tumor were used. For patients who underwent radiotherapy or concurrent chemoradiotherapy (CCRT), pathologic specimens were only one or two for biopsy, therefore, microdissection was performed to ensure purity of tumor cell. DNA was then extracted from paraffin-embedded tissue and amplified by PCR. For HPV DNA detection and genotyping, MyHPV Chip kit (Mygene Co., Seoul, Korea) was used, containing 24 type-specific oligonucleotide probes from high-risk (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 54, 56, 58, 59, 66, and 68) and low-risk types (6, 11, 34, 40, 42, 43, 44, and 70). HPV infection was defined by high expression of p16. For IHC staining, CINtec Histology kit (MTM Laboratories AG, Heidelberg, Germany), anti-p53 (Invitrogen, Camarillo, CA, USA), and Novocastra anti-EGFR (Leica Biosystems, Newcastle, UK) were used.
Immunoreactivity for p16, p53, and EGFR were defined as 'high' or 'low' depending on the staining intensity. For p16, strong nuclear and cytoplasmic staining in more than 60% of tumor cells were defined as high [
4]. For p53, nuclear immunoreactivity in more than 50% of tumor cells were defined as high [
11]. For EGFR, membrane staining in more than 50% of cancer cells was considered as high [
4,
12]. These evaluations were carried out semiquantitatively by one pathologist blinded to the clinical outcome (
Fig. 1).
 | Fig. 1Immunohistochemical staining of p16, p53, epidermal growth factor receptor (EGFR), and Rb. All photographs are taken in high power field (×400). (A) High expression of p16, (B) low expression of p16, (C) high expression of p53, (D) low expression of p53, (E) high expression of EGFR, (F) low expression of EGFR, (G) high expression of Rb, and (H) low expression of Rb. 
|
Statistical analysis
The relationship between HPV status and clinical parameters or expression of molecular markers was analyzed using the Fisher exact test or the chi-square test. The Kaplan-Meier method and the log-rank or Breslow's test were used to compare survival rates. For adjusting T stage, we categorized the patient into subgroups on the basis of T stage (T1-2 vs. T3-4). For univariable and multivariable analysis, Cox regression models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). Two-tailed
P-values <0.05 were considered significant. Statistical analysis used SAS ver. 9.1.3 (SAS Institute, Cary, NC, USA) and R 2.13.2 (Vienna, Austria;
http://www.R-project.org) software.
Go to :

RESULTS
Characteristics of patients
The mean age was 63.4 years (range, 43 to 81 years). There were 25 men and 8 women. The mean follow-up period was 62.4 months (range, 9 to 99 months). Two patients received surgery alone, 19 patients were treated with surgery and postoperative radiotherapy. Thus surgery-based treatment was conducted in 21 (63.6%) patients. Radiation-based treatment was conducted in 12 (36.4%) patients; radiation alone in 2 patients, and CCRT in 10 patients.
There were 6 (18.1%) p16 low expression and 27 (81.9%) p16 high expression tonsil cancers. There were no statistically significant differences between high and low expression groups with respect to sex, age, smoking history, T stage, N stage, and treatment methods (
Table 1). Although it is not statistically significant, p16 high expression patients likely had more advanced staged cancers (
P=0.078).
Table 1
Association of p16 status and clinicopathological findings


Association between HPV status and immunohistochemical markers
All HPV DNA-detected cases had type 16 infection except one (type 6). High expression rates of IHC in p16, p53, and EGFR were 81.8%, 24.2%, and 33.3%, respectively. High expression rates of p53, EGFR, and HPV DNA were not associated with p16 immunoreactivity (
Table 1).
Disease-free survival (DFS) according to the p16
In T1-2 stage, the 5-year DFS of p16 low expression patients was 50%, compared with 80.0% for p16 high expression patients. In T3-4 stage, the 5-year DFS of p16 low expression patients was not assessed, compared with 58.3% for p16 high expression patients.
Fig. 2 shows the DFS curves of the each groups according to p16, which were not significantly different (
P=0.053).
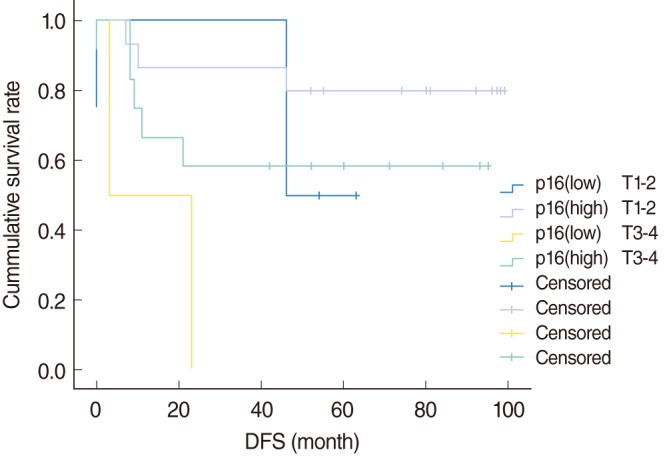 | Fig. 2Disease-free survival (DFS) curves according to p16 expression. 
|
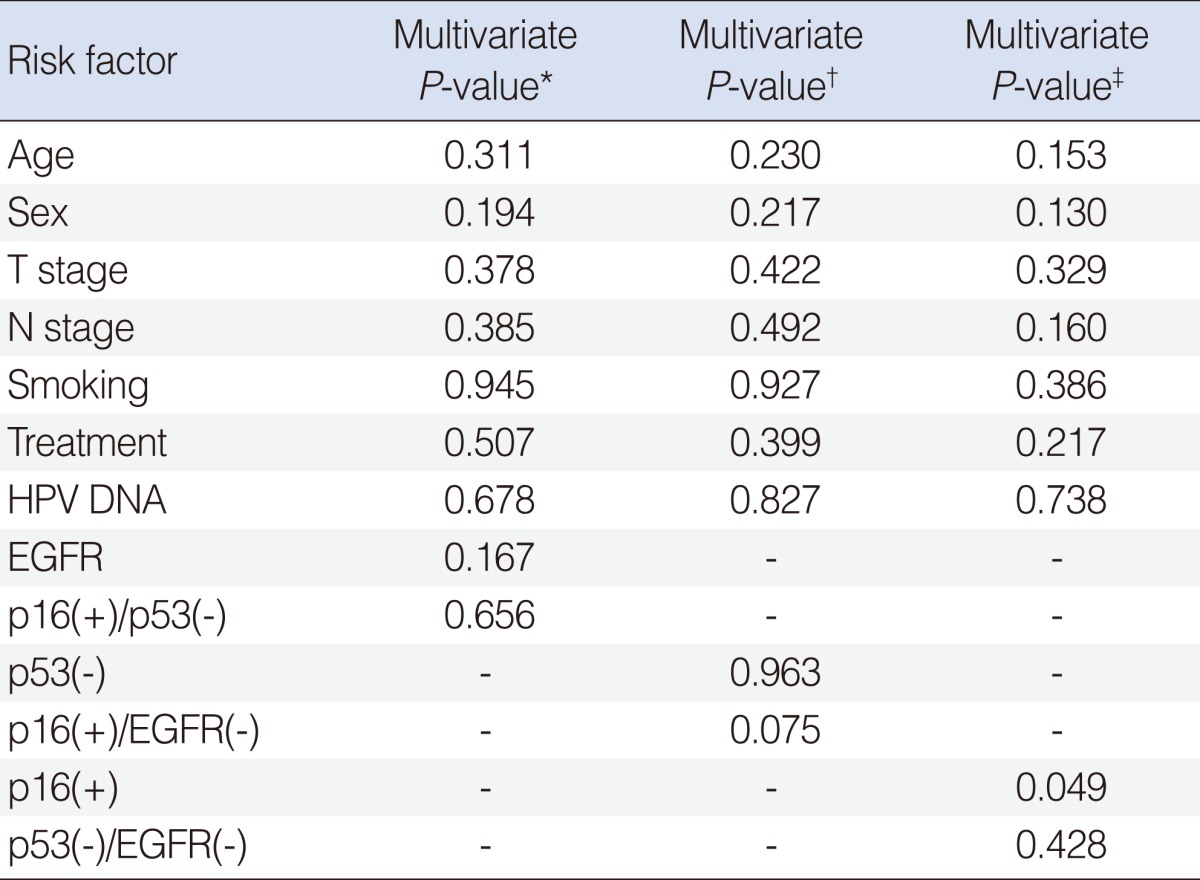
Univariate and mutivariate analysis of clinicopathological variables for DFS
Univariate analysis using Cox regression model identified EFGR (
P=0.041) as a risk factor for DFS, consistent with previous reports (
Table 2). Univariate analysis did not find other associations with DFS. We then performed a multivariate analysis to assess the factors affecting the recurrence rates among the considerable risk factors. Relative HR, 95% CI, and
P-value of each possible risk factor are also listed in
Table 2. Adjusting for other variables, we found that p16 was associated with higher risk of recurrence (HR, 9.53; 95% CI, 1.04 to 87.53;
P=0.046). Since combinations of IHC markers were derivatives of p16, p53, and EGFR, these should not be included in the same models, to avoid multicollinearity. Therefore, Cox regression analysis was performed using 3 different models. In these three models, no combinations of IHC markers showed statistical significance for survival (
Table 3).
Table 2
Univariate and mutivariate analyses, Cox regression of recurrence-free survival


Table 3
Multivariate analysis for combinations of p16, p53, and EGFR for recurrence-free survival

Go to :

DISCUSSION
Roughly 50 studies have investigated the effect of HPV on HNSCC [
3]. It is well established that the HPV-positive patients have better survival compared with HPV-negative patients, but some studies suggest that HPV infections have no effect on survival or may even predict a poor prognosis [
3].
In a meta-analysis reported in 2007, only nine out of 33 articles demonstrated no survival advantage in patients with HPV-associated tumors, and 3 studies indicated negative association between HPV positivity and survival [
13]. In this meta-analysis of 2,538 patients of HNSCC, 1,024 patients had OPSCC. HPV-associated OPSCC had 28% reduced risk of death and 49% reduced risk of disease failure compared to HPV-negative OPSCC, whereas in non-OPSCC, no differences in survival were observed between HPV-positive and HPV-negative patients [
13].
In our study, patients with tonsil cancer were reviewed because HPV DNA is known to be detected most frequently in this tumor and associations between HPV status and prognosis have been reported as relatively consistent in tonsil cancer.
Recent studies [
9,
10] have reported strong association between integrated HPV viral detection and p16 protein over-expression in oropharyngeal cancer, suggesting that p16 IHC is a more effective method for identification of HPV-associated oropharyngeal cancer in clinical practice. This is mainly due to simplicity, low cost, and the feasibility of the IHC analysis. To increase clinical practice applicability, we defined HPV infection by high expression of p16.
Our results showed no association of HPV DNA and p16 high expression rate. We found a p16 high expression rate of 86.7% in HPV DNA(+) patients in our specimens. Statistical power was lost because of p16 high expression (77.8%) also in HPV DNA(-) patients (
Table 1). Smeets et al. [
14] reported that only 50% of the similar specimens were PCR(+) in formalin-fixed paraffin-embedded tissue. Compared to PCR, IHC including p16 has relatively consistent sensitivity in both paraffin block and fresh frozen tissue. We postulate that lack of association of HPV DNA and p16 expression was primarily due to different sensitivity of methods according to the specimens.
The current diagnostic tools to detect HPV DNA are mostly based on PCR. These methods demonstrate only the presence of HPV DNA, and cannot differentiate whether the HPV DNA has been found in the tumor or in surrounding normal tissue, and whether this viral DNA initiated oncogenesis.
Therefore, we also reasoned that expression of p16 could be more valuable as a prognostic factor than HPV DNA itself. In order to demonstrate this hypothesis, multivariate modelling including HPV DNA status and p16 was performed (
Table 2). In this Cox regression model, HPV DNA was not significantly associated with recurrence rates adjusting for other variables, although p16 expression was borderline significantly associated with the recurrence rates (
P=0.046). The relative HR was 9.53 (95% CI, 1.04 to 87.53) and supported by Kaplan-Meier survival curves (
Fig. 2).
A p53 is a representative tumor suppressor protein, and half or more of HPV-negative HNSCC are known to have an identifiable p53 mutation. However, p53 mutation in HPV-positive HNSCC is rare, and E6 protein produced by HPV binds to p53 and marks it for degradation via a proteasome-dependent pathway [
3].
So it is generally accepted that in HPV-positive HNSCC, expression of p53 would be low and some studies have indeed reported low expression of p53 in HPV-positive HNSCCs [
7]. However, over-expressions of p53 in HPV-positive HNSCC have also been reported [
15,
16]. We found the expression rate of p53 was 18.5% in p16 high expression patients, compared with 50.0% in p16 low expression patient (
P=0.137). Little is known about the meaning of this association. It is sure that p53 mutation in HPV-infected HNSCC is rare and better prognosis of HPV-infected HNSCC is partially attributed to this fact. Because p53 induces apoptosis in the presence of cytotoxic stress including chemotherapy and radiotherapy, the presence of wild type p53, regardless of the expression rate, is likely to lead to better prognosis in HPV-infected patients.
EGFR is known to be strongly expressed in HNSCC and over-expressed EGFR is also known to be related to poor prognosis. Our data showed that low expression of EGFR was observed in 22 (66%) of total patient and significantly associated with the recurrence rates in univariate analysis (P=0.041). But EGFR did not maintain its statistical significance after multivariate adjustment.
It was recently suggested that combined IHC is both easily applicable and provides important prognostic information for HNSCC. Reimers et al. [
4] found that the comparison of the p16(+)/EGFR(-) group with the p16(-)/EGFR(+) group revealed a significant difference in survival rate. Three different models were estimated to investigate how combination of p16, p53 and EGFR is associated with DFS using Cox regression model in this study. Any association wasn't found in the first model including EGFR and combination of high p16 and low p53, and in the second model including p53 and combination of high p16 and low EGFR. Significance of combination of low p53 and low EGFR was not found, but p16 was significantly associated with DFS in the third model (
Table 3).
There are several limitations in this study. First, only 33 patients with tonsil cancer were involved in this study. In addition, patients' distribution between HPV-positive and negative groups was not even, due to the small number of patients and retrospective design, possibly affecting recurrence rates. Second, paraffin-embedded specimens are known to have poorer HPV detection than fresh specimens. Transcriptase-PCR or in situ hybridization as a reference standard was not used for confirming viral integration. Finally, the general status of immunohistochemical staining was not satisfactory in some cases.
Go to :

ACKNOWLEDGMENTS
This study was supported by a grant from the Korean Health Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A100862).
Go to :

Notes
Go to :

References
1. Syrjanen K, Syrjanen S, Lamberg M, Pyrhonen S, Nuutinen J. Morphological and immunohistochemical evidence suggesting human papillomavirus (HPV) involvement in oral squamous cell carcinogenesis. Int J Oral Surg. 1983; 12. 12(6):418–424. PMID:
6325356.
2. Ha PK, Califano JA. The role of human papillomavirus in oral carcinogenesis. Crit Rev Oral Biol Med. 2004; 7. 15(4):188–196. PMID:
15284184.

3. Allen CT, Lewis JS Jr, El-Mofty SK, Haughey BH, Nussenbaum B. Human papillomavirus and oropharynx cancer: biology, detection and clinical implications. Laryngoscope. 2010; 9. 120(9):1756–1772. PMID:
20669304.

4. Reimers N, Kasper HU, Weissenborn SJ, Stutzer H, Preuss SF, Hoffmann TK, et al. Combined analysis of HPV-DNA, p16 and EGFR expression to predict prognosis in oropharyngeal cancer. Int J Cancer. 2007; 4. 120(8):1731–1738. PMID:
17236202.

5. Hafkamp HC, Manni JJ, Haesevoets A, Voogd AC, Schepers M, Bot FJ, et al. Marked differences in survival rate between smokers and nonsmokers with HPV 16-associated tonsillar carcinomas. Int J Cancer. 2008; 6. 122(12):2656–2664. PMID:
18360824.

6. Gillison ML, Harris J, Westra W, Chung C, Jordan R, Rosenthal D, et al. Survival outcomes by tumor human papillomavirus (HPV) status in stage III-IV oropharyngeal cancer (OPC) in RTOG 0129 [abstract]. J Clin Oncol. 2009; 27(15S):6003.
7. Al-Swiahb JN, Huang CC, Fang FM, Chuang HC, Huang HY, Luo SD, et al. Prognostic impact of p16, p53, epidermal growth factor receptor, and human papillomavirus in oropharyngeal cancer in a betel nut-chewing area. Arch Otolaryngol Head Neck Surg. 2010; 5. 136(5):502–508. PMID:
20479383.

8. Ciardiello F, Tortora G. A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin Cancer Res. 2001; 10. 7(10):2958–2970. PMID:
11595683.
9. Thomas J, Primeaux T. Is p16 immunohistochemistry a more cost-effective method for identification of human papilloma virus-associated head and neck squamous cell carcinoma? Ann Diagn Pathol. 2012; 4. 16(2):91–99. PMID:
22197546.

10. El-Naggar AK, Westra WH. p16 expression as a surrogate marker for HPV-related oropharyngeal carcinoma: a guide for interpretative relevance and consistency. Head Neck. 2012; 4. 34(4):459–461. PMID:
22180304.

11. Chien CY, Huang CC, Cheng JT, Chen CM, Hwang CF, Su CY. The clinicopathological significance of p53 and p21 expression in squamous cell carcinoma of hypopharyngeal cancer. Cancer Lett. 2003; 11. 201(2):217–223. PMID:
14607337.

12. Li W, Tran N, Lee SC, O'Brien CJ, Tse GM, Scolyer RA, et al. New evidence for geographic variation in the role of human papillomavirus in tonsillar carcinogenesis. Pathology. 2007; 4. 39(2):217–222. PMID:
17454751.

13. Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007; 10. 121(8):1813–1820. PMID:
17546592.

14. Smeets SJ, Hesselink AT, Speel EJ, Haesevoets A, Snijders PJ, Pawlita M, et al. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer. 2007; 12. 121(11):2465–2472. PMID:
17680565.

15. Hafkamp HC, Speel EJ, Haesevoets A, Bot FJ, Dinjens WN, Ramaekers FC, et al. A subset of head and neck squamous cell carcinomas exhibits integration of HPV 16/18 DNA and overexpression of p16INK4A and p53 in the absence of mutations in p53 exons 5-8. Int J Cancer. 2003; 11. 107(3):394–400. PMID:
14506739.

16. Kumar B, Cordell KG, Lee JS, Worden FP, Prince ME, Tran HH, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008; 7. 26(19):3128–3137. PMID:
18474878.

Go to :







 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download