Abstract
Objectives
The stimulation levels programmed in cochlear implant systems are affected by an evolution since the first switch-on of the processor. This study was designed to evaluate the changes in stimulation levels over time and the relationship between post-implantation physiological changes and with the hearing experience provided by the continuous use of the cochlear implant.
Methods
Sixty-two patients, ranging in age from 4 to 68 years at the moment of implantation participated in this study. All subjects were implanted with the 12 channels COMBI 40+ cochlear implant at San Cecilio University Hospital, Granada, Spain. Hearing loss etiology and progression characteristics varied across subjects.
Results
The analyzed programming maps show that the stimulation levels suffer a fast evolution during the first weeks after the first switch-on of the processor. Then, the evolution becomes slower and the programming parameters tend to be stable at about 6 months after the first switch-on. The evolution of the stimulation levels implies an increment of the electrical dynamic range, which is increased from 15.4 to 20.7 dB and improves the intensity resolution. A significant increment of the sensitivity to acoustic stimuli is also observed. For some patients, we have also observed transitory changes in the electrode impedances associated to secretory otitis media, which cause important changes in the programming maps.
Conclusion
We have studied the long-term evolution of the stimulation levels in cochlear implant patients. Our results show the importance of systematic measurements of the electrode impedances before the revision of the programming map. This report also highlights that the evolution of the programming maps is an important factor to be considered in order to determine an adequate calendar fitting of the cochlear implant processor.
A cochlear implant is a device designed to generate an electrical stimulation of the auditory nerve, and provides hearing perception to patients affected by severe and profound hearing loss. Currently, intra-cochlear multichannel implants allow the perception of the audio signal with enough quality to understand speech in most cases [1,2]. The cochlear implant divides the audio signal into spectral bands, each of them associated to a channel. The audio dynamic range in each band is mapped into the electrical dynamic range defined by two reference electrical levels associated to the corresponding channel: the electrical threshold (THR) or minimum electrical level the patient can perceive, and the maximum comfortable level (MCL) or maximum level the patient accepts without uncomfortable sensation. Cochlear implant systems must be programmed in order to adapt the electrical stimulation levels of each channel to the requirements of the patient. The programming of a cochlear implant is essentially to estimate the THR and the MCL levels for each channel. An accurate estimation of the THR and MCL levels is very important to enable proper perception using the cochlear implant [3]. The estimation of these parameters is usually obtained from the subjective responses to a series of stimuli presented at each electrode at different levels [3,4]. Therefore, a proper programming of the cochlear implant requires patient collaboration. In the case of poor collaboration, some authors propose the use of information obtained from objective measurements [5-11], indirect observation of the responses to acoustic stimulation [12,13] or statistical analysis of the programming maps [14].
The programming parameters are affected by an evolution since the first switch-on of the processor onward. The continuous stimulation of the cochlear nerve increases the neural activity and produces the recruiting of neurons in the neighborhood of each active electrode. This increases the sensitivity of the cochlear nerve and makes easier the generation of a response to a low level stimulation. The evolution of the THR and MCL levels has been previously reported by Schmidt and Griesser [15]. The programming parameters changed very fast during the first month after the switch-on. Then the evolution slows progressively and finally the programming maps become stable for most patients. Hughes et al. [16] showed that MCL levels in the Nucleus device were not stable until 12 months after cochlear implant activation. Henkin et al. [17] found the stabilization of THR levels after 3 months and MCL levels after 6 months of implant use. Brown et al. [18] and Franck and Norton [19] reported that THR and MCL levels have generally stabilized by approximately 3 months after connection.
In this paper, we analyze the evolution of the programming parameters for a group of patients implanted in our ENT service. In addition, we analyze the evolution of the sensitivity to acoustic stimuli (which evaluates the progress in the hearing experience), the evolution of electrode impedance (which is related to the intra-cochlear fibrosis), and the relationship between these facts and the evolution of the THR and the MCL levels. The insertion of the electrode carrier inside the cochlea produces a progressive intra-cochlear fibrosis. The fibrosis has been detected during surgery in all the cases of cochlear re-implantation and is supposed to affect all the implanted patients. The intra-cochlear fibrosis produces an increment of electrical impedances of the electrodes and this causes a modification of the stimulation levels the patient needs. On the other hand, there is an increment in the tolerance to high stimulation levels and better sensitivity to low electrical stimuli as the patient is more experienced in the use of the cochlear implant. All these facts modify the electrical THRs and the MCLs corresponding to each electrode and make the re-programming of the THR and MCL parameters necessary.
In order to clarify the relation between the modification of the programming maps and the modifications of the electrical impedances, we report transitory alterations in the impedances and the stimulation levels detected for some of the implanted patients. These transitory changes were associated with secretory otitis media and motivated a loss of quality in the hearing perception of the affected patients until the processors were re-programmed with proper stimulation levels. The results about the evolution of the stimulation levels provide information about a schedule of revisions for persons with cochlear implants.
We implanted 12 channel COMBI 40+ (MED-EL, Bridgend, UK) cochlear implants in 62 patients at our ENT service. We selected patients that had a cochlear implant for more than 12 months. We excluded patients that could not perform pure tone audiometry (PTA) from enrolling into this study. At the moment of implantation, the patients ranged in age from 4 to 68 years (23 of them were implanted younger than 7 years, 20 were between 8 and 17 years, and 19 were implanted older than 18 years). The patients had been affected by different degrees of hearing-loss, with different durations and etiology. The study was approved by the Internal Review Board of San Cecilio University Hospital.
To study the long-term evolution in time of the stimulation levels, we have analyzed the programming maps obtained during the fitting sessions. The THR and MCL levels have been estimated from the subjective responses to series of stimuli presented to the patients at different electrodes and with different levels. During the first weeks after the first switch-on, the evolution of the perception capability is evaluated by means of behavioral or PTA, speech perception, discrimination and understanding tests, and other indirect methods. The audiologist used this information, as references for an accurate estimation of the THR and MCL levels. In the COMBI 40+ device (MED-EL), the stimulation levels are obtained by combining the electrical intensity (i) and the duration (t) of each phase of the biphasic pulses generated by the implant. The loudness sensation depends on the product (i×t), i.e., the total charge (q) inserted in each phase of the pulse. For this reason, we have expressed the stimulation levels in charge units (nano-Coulombs, nC) in order to be able to compare stimulation levels with different intensities and durations. In this study, we are interested on the evolution in time of the stimulation levels. For this reason, we have averaged the THR and MCL levels estimated for each active electrode. The evolution in time has been analyzed taking into account the averaged THR and MCL levels obtained during the fitting sessions 2 weeks, 1 month, 2, 3, 4, 6, 8, 10, and 12 months after the first switch-on of the cochlear implant processor.
During the fitting sessions we have also obtained audiometric measurements. The hearing sensitivity has been measured by means of open field aided PTA for the frequencies 250, 500, 1,000, 2,000, and 4,000 Hz. For each patient and each fitting session we averaged the sensitivity measured for the different frequencies. In this manner, we have analyzed the long-term evolution of the hearing sensitivity provided by the cochlear implant as well.
The COMBI 40+ (MED-EL) cochlear implant includes the technical utilities to check the integrity and functionality of the implant. These tools allow the estimation of the electrical impedance of each electrode. The impedance of the electrodes determines the efficiency of the current insertion and is related to the conductivity of the intra-cochlear medium. The low impedance means a good conductivity and an efficient insertion of current from the electrodes. In this case, the electrical stimulation is efficient and the THR and MCL levels necessary for a proper stimulation are usually low. During the fitting sessions, we have measured the electrode impedances and we have studied the evolution of the impedance averaged over all the active electrodes for each patient. In order to analyze the relation between the changes in the impedance and the changes in the stimulation levels, we have analyzed the long-term evolution of the electrode impedances.
The THR and MCL parameters are affected by an evolution as shown in Fig. 1. This plot represents the evolution in time of the averaged THR and MCL levels (the error bars represent mean±standard deviation). An important increment in the MCL levels was observed during the first month and afterwards, the evolution was slower. The MCL levels reached stable values at approximately 6 months after the first switch-on. The average MCL level was increased by 54% during the period between 2 weeks and 6 months. A Welch test [20] has been applied to analyze the significance of these results. MCL levels increased significantly from initial value to the 12-month time-point (P=1.70e-9). Comparisons between consecutive time points revealed significant differences between levels at 0.5 and 1 month (P=1.80e-3), those at 0.5 and 6 months (P=9.44e-10), and those at 1 and 6 months post initial stimulation (P=1.8e-3). No significance differences were found between 6 and 12 months. We have also analyzed the evolution of MCL levels dividing the months in 3 segments: initial segment (months 0.5, 1, and 2), medium segment (months 3, 4, and 6), and final segment (months 8, 10, and 12). We found that MCL levels increased significantly from initial to the final segment (1.42e-9), but no statistically significant differences were found when comparing medium and final segment.
The THR level was also affected by an evolution. During the first weeks, a small value was observed compared to the rest of the evolution. During this period, the THR based on subjective responses tends to be over-estimated due to the lack of recent hearing experience in most of the patients. For this reason, during the first fitting sessions the processors are usually programmed with THR levels significantly lower than those estimated from the subjective responses. About one month after the first switch-on, the patients are experienced enough to accurately estimate THR levels based on the subjective responses. This bias, artificially introduced by the audiologist, causes the reduced THR level observed 2 weeks after the first switch-on. Since the first month onward, the THR levels tended to be reduced and stable values were reached at about 6 months after the first switch-on. During this period, the THR levels were reduced by 22%. Since the THR value at 0.5 month present an artificial value, it has been excluded for the statistical analysis. THR levels decreased significantly when comparing levels at 1 month and 12 months, but with a high value of P (P=4.01e-2). THR levels in the initial segment (months 1 and 2) were significantly higher than those in the final segment (P=8.56e-3), but there were not statistically significant differences between the medium and final segments.
The global tendencies observed for the studied group of patients is similar to that reported by Schmidt and Griesser [15]; for the stable averaged stimulation levels, we have observed a lower THR (they obtained 4.0 nC and we obtained 2.8 nC) and a greater MCL (they obtained 24.0 nC and we obtained 30.5 nC). We obtained smaller average standard deviations. These differences could be associated to the sample of patients considered in both studies and the differences in the device utilized (the patients in the other study had been implanted with the 8 electrode COMBI 40+ [MED-EL] cochlear implant).
The evolution of the programming parameters shows an increment in the tolerance to higher stimulation levels. On the other hand, the sensitivity to stimuli with lower intensity is improved as well. The result of the evolution of both, the THR and MCL levels, is an increment of the electrical dynamic range as the experience in the use of the cochlear implant is increased. The average dynamic range evolved from 15.4 dB (2 weeks after the first switch-on) to 20.7 dB (when the stability was reached). This evolution increases the intensity resolution (i.e., the ability for the discrimination between stimuli with different intensity is improved), which plays an important role in the quality of the hearing perception and the speech recognition.
The evolution of the hearing sensitivity measured from aided PTA is shown in Fig. 2. The error bars represent the averaged sensitivity±standard deviation. In this plot, a fast increment of the sensitivity was observed during the first months and the stabilization was reached at about 6 months after the first switch-on. The sensitivity at 6 months was statistically significantly better than initial test value (P<1e-16) and no significant differences were found between 6 and 12 months. The average improvement observed between the first switch-on and the stabilization was of about 14.0 dB. This improvement is associated to the hearing experience provided by the cochlear implant (which allows the patients to identify stimuli with a lower intensity) and the reactivation of the auditory pathway due to the continuous stimulation. We have observed that those patients affected by longer duration and more profound hearing-losses before being implanted (more affected by the retrograde degeneration of the cochlear nerve), presented worse sensitivity during the first weeks (with thresholds close to 50 dB hearing threshold level [HTL]).
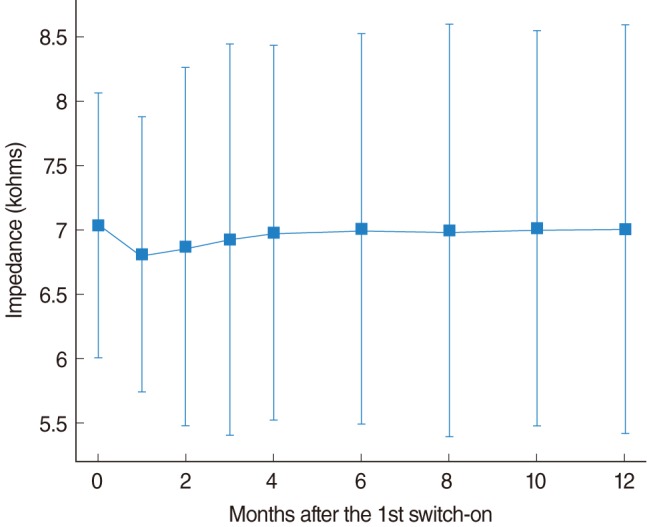
The evolution of the impedance is shown in Fig. 3. A slight reduction in the impedances was observed during the first weeks and then, there was a progressive increment that reached the stability at about 6 months after the first switch-on. The high impedance at the beginning is caused by air bubbles trapped close to the electrodes during the insertion of the electrode carrier. These bubbles reduce the effective surface of the electrodes and increase the electrode impedances. The air bubbles are absorbed during the first weeks, which produce the impedance reduction observed in this plot.
The increment of the averaged impedances observed in the next months can be associated with the progressive intra-cochlear fibrosis caused by the electrode carrier. The fibrosis (observed in the cases of re-implantation and probably affecting to all the patients) produces and alteration of the electric properties of the intra-cochlear medium, which causes the progressive increment of impedances. Although impedances tended to increase with the use of the cochlear implant, no statistically significant differences were found between months due to the high inter-patient variability. The increment was small (0.2 kohm) compared to the standard deviation of the impedances distribution observed for the implanted patients.
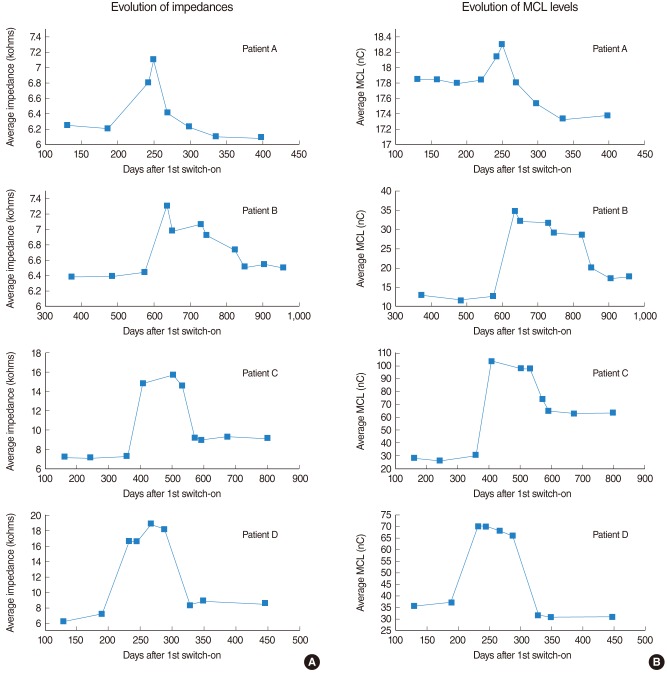
In 4 of the patients considered, we observed an important increment in the impedances differed from the evolution described in the previous section. In these cases, the increment in impedance was associated with secretory otitis media and an accumulation of mucus in the middle ear. The alteration in impedance was accompanied by an increment in the requested stimulation levels and new re-fittings were necessary in order to adapt the programming maps to these changes. As the disease disappeared with the proper treatment, the impedances were reduced, as well as the stimulation levels.
Fig. 4 shows the evolution of the impedances (plots in the left side) and the evolution of the MCL levels (in the right side) corresponding to the specific patient groups. Patient A was affected by moderate changes in both, the impedances and the stimulation levels. Patients C and D were affected by very important changes in both, and levels near 3 times the ones previous to the alterations were reached. In the case of patient B, an important increment in the MCL levels is observed even though the impedances suffered a moderate increment. After the disease disappeared, the impedances and the stimulation levels were significantly reduced, reaching values relatively similar to the initial ones. A high correlation between the changes in the impedances and the changes in the MCL levels can be observed by comparing the plots associated to each patient.
The presented results show that the evolution of the stimulation levels keeps a close relation to physiological changes, as well as to the experience in the use of the cochlear implant. The analysis of the transitory alterations associated with secretory otitis media show the important role played by the electrode impedance over the increment observed in the stimulation levels. The progressive increment in the impedances associated to the intra-cochlear fibrosis during the months after the first switch-on, could be one of the most important factors causing the observed increment in the MCL levels. However, the impedance increment cannot completely explain the evolution of the programming maps. First, the observed evolution of the impedances is not statistically significant. Second, the increment of impedances cannot justify the reduction in the THR levels. And finally, the increment in the hearing sensitivity parallel to the reduction of the THR levels clearly shows a significant reactivation of the auditory pathway and the auditory cortex due to the continuous stimulation provided by the cochlear implant. The results presented in this paper show that the experience in the use of the cochlear implant improves the functionality of the auditory pathway; this includes the recruiting of neurons in the neighborhood of the active electrodes and the reduction of the activation THRs of the neurons. The continuous experience in the use of the cochlear implant makes the patients better able to tolerate more intense stimuli, as well as to detect less intense stimuli. This factor also contributes to the evolution of the programming maps.
The knowledge of programming maps evolution is useful for planning the fitting sessions. During the first weeks, due to the lack of experience, the estimation of stimulation levels based on subjective responses is affected by an important bias. For this reason, during the first two weeks, it would be recommendable to review the programming maps several times in order to estimate reliable stimulation levels. After this period, we think new fitting sessions at 1, 3, 6, and 12 months after the first switch-on should be enough for adapting the programming maps to the physiological changes and the increasing hearing experience of the patients. After the first year, a visit every 6 months for technical checking, impedance measurement, and revision of the THR and MCL levels is recommended.
In the case of transitory alteration of the impedances (like those described in the cases of secretory otitis media), the patient would need higher stimulation levels. For this reason, in the case of adults, they ask for a revision, and in the case of children, a significant reduction of the perception abilities is detected and the parents ask for the revision. A systematic control of the evolution of the impedances is necessary in order to detect these cases. If the patient is affected by important changes in the stimulation levels or the impedances, several fitting sessions must be considered in order to measure the evolution of the impedances and to adapt the stimulation parameters to the requirements of the patient.
In conclusion, we have studied the long-term evolution of the stimulation levels in cochlear implant patients, and its relation with post-implantation physiological changes and with the hearing experience provided by the continuous use of the cochlear implant. After the first switch-on of the processor, the MCL levels suffer an average increment of 54% and the THR levels an average reduction of 22%. The stable levels are reached at about 6 months after the first switch-on for most of the patients. The evolution of the stimulation levels implies an increment of the electrical dynamic range, which is increased from 15.4 to 20.7 dB which improves the intensity resolution. A significant increment in the sensitivity to acoustic stimuli is observed as well (there is an improvement of about 14.0 dB).
In some patients, we observed transitory alterations of the electrode impedances associated to secretory otitis media. These alterations were accompanied by important changes in the stimulation levels and made necessary the revision of the programming map. These observations show the importance of systematic measurements of the electrode impedances.
The results presented in this paper show the tendency of the programming maps, since the first switch-on until a stable value is obtained. In addition, we have analyzed the main causes affecting the evolution of the stimulation levels. The observed evolution allows for proper planning of fitting sessions.
ACKNOWLEDGMENTS
The authors thank the contribution made by patients and members of the Cochlear Implant Program at the ENT Service of San Cecilio University Hospital, Granada, Spain.
References
1. Wilson BS, Finley CC, Lawson DT, Wolford RD, Eddington DK, Rabinowitz WM. Better speech recognition with cochlear implants. Nature. 1991; 7. 352(6332):236–238. PMID: 1857418.

3. Dawson PW, Skok M, Clark GM. The effect of loudness imbalance between electrodes in cochlear implant users. Ear Hear. 1997; 4. 18(2):156–165. PMID: 9099565.

4. Battmer RD. Fitting in the real world: all the practical questions and problems. In : The 5th European Symposium on Paediatric Cochlear Implantation; 2000 Jun 4-7; Antwerp, Belgium.
5. Hodges AV, Balkany TJ, Ruth RA, Lambert PR, Dolan-Ash S, Schloffman JJ. Electrical middle ear muscle reflex: use in cochlear implant programming. Otolaryngol Head Neck Surg. 1997; 9. 117(3 Pt 1):255–261. PMID: 9334774.

6. Brown CJ, Hughes ML, Lopez SM, Abbas PJ. Relationship between EABR thresholds and levels used to program the CLARION speech processor. Ann Otol Rhinol Laryngol Suppl. 1999; 4. 177:50–57. PMID: 10214802.

7. Stephan K, Welzl-Muller K. Post-operative stapedius reflex tests with simultaneous loudness scaling in patients supplied with cochlear implants. Audiology. 2000; Jan-Feb. 39(1):13–18. PMID: 10749066.

8. Han DM, Chen XQ, Zhao XT, Kong Y, Li YX, Liu S, et al. Comparisons between neural response imaging thresholds, electrically evoked auditory reflex thresholds and most comfortable loudness levels in CII bionic ear users with HiResolution sound processing strategies. Acta Otolaryngol. 2005; 7. 125(7):732–735. PMID: 16012035.

9. King JE, Polak M, Hodges AV, Payne S, Telischi FF. Use of neural response telemetry measures to objectively set the comfort levels in the Nucleus 24 cochlear implant. J Am Acad Audiol. 2006; 6. 17(6):413–431. PMID: 16866005.

10. Holstad BA, Sonneveldt VG, Fears BT, Davidson LS, Aaron RJ, Richter M, et al. Relation of electrically evoked compound action potential thresholds to behavioral T- and C-levels in children with cochlear implants. Ear Hear. 2009; 2. 30(1):115–127. PMID: 19125034.

11. Alvarez I, de la Torre A, Sainz M, Roldan C, Schoesser H, Spitzer P. Using evoked compound action potentials to assess activation of electrodes and predict C-levels in the Tempo+ cochlear implant speech processor. Ear Hear. 2010; 2. 31(1):134–145. PMID: 19838116.

12. Shallop JK, Ash KR. Relationships among comfort levels determined by cochlear implant patient's self-programming, audiologist's programming, and electrical stapedius reflex thresholds. Ann Otol Rhinol Laryngol Suppl. 1995; 9. 166:175–176. PMID: 7668623.
13. Madell J, Ozdamar S, Sislian N, Hoffman R. Using speech perception errors to modify programming. In : The 8th Symposium on Cochlear Implants in Children; 2001 Feb 28-Mar 3; Los Angeles, CA, USA.
14. Sainz M, de la Torre A. Perceptual thresholds of the electrical impulses in cochlear implanted patients. In : The 8th Symposium on Cochlear Implants in Children; 2001 Feb 28-Mar 3; Los Angeles, CA, USA.
15. Schmidt M, Griesser A. Long-term stability of fitting parameters with the COMBI 40. Am J Otol. 1997; 11. 18(6 Suppl):S109–S110. PMID: 9391621.
16. Hughes ML, Vander Werff KR, Brown CJ, Abbas PJ, Kelsay DM, Teagle HF, et al. A longitudinal study of electrode impedance, the electrically evoked compound action potential, and behavioral measures in nucleus 24 cochlear implant users. Ear Hear. 2001; 12. 22(6):471–486. PMID: 11770670.

17. Henkin Y, Kaplan-Neeman R, Muchnik C, Kronenberg J, Hildesheimer M. Changes over time in electrical stimulation levels and electrode impedance values in children using the Nucleus 24M cochlear implant. Int J Pediatr Otorhinolaryngol. 2003; 8. 67(8):873–880. PMID: 12880667.

18. Brown CJ, Hughes ML, Luk B, Abbas PJ, Wolaver A, Gervais J. The relationship between EAP and EABR thresholds and levels used to program the nucleus 24 speech processor: data from adults. Ear Hear. 2000; 4. 21(2):151–163. PMID: 10777022.

19. Franck KH, Norton SJ. Estimation of psychophysical levels using the electrically evoked compound action potential measured with the neural response telemetry capabilities of Cochlear Corporation's CI24M device. Ear Hear. 2001; 8. 22(4):289–299. PMID: 11527036.

Fig. 1
Evolution of the averaged stimulation levels after the first switch-on of the processor. The vertical bars represent the mean±standard deviation. Stabilization occurred after 6 months of implant use. THR, threshold; MCL, maximum comfortable level; nC, nano-Coulombs.
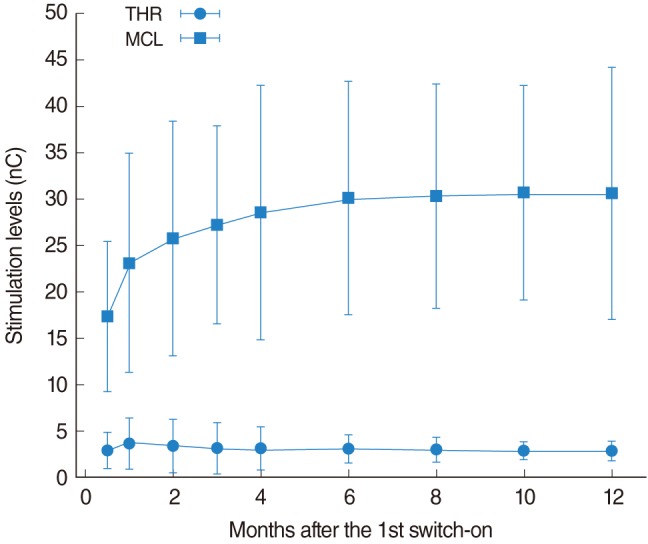
Fig. 2
Evolution of the hearing sensitivity obtained from aided open-field pure tone audiometry. The error bars represent averaged hearing sensitivity±standard deviation. Hearing sensitivity increased during the first 6 months of implant use, thereafter stabilization occurred.
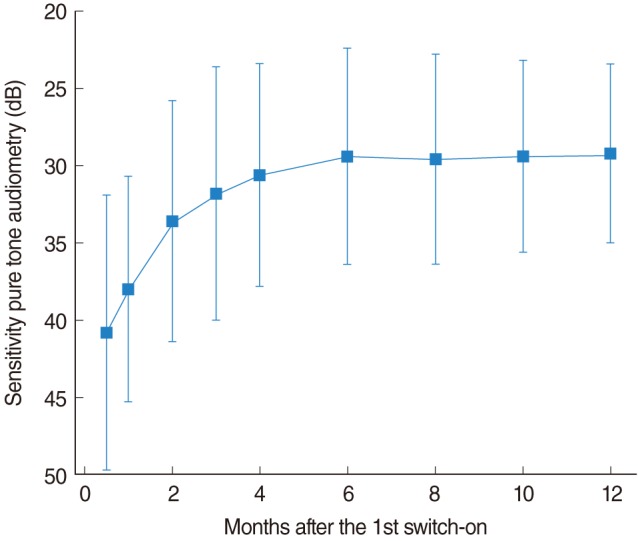
Fig. 3
Evolution of the average electrode impedance. The vertical bars represent the mean±standard deviation. A reduction in the averaged impedances was observed during the first weeks. Although impedances tended to increase with the use of the cochlear implant, no statistically significant differences were found between months.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download