Abstract
Objectives
To assess the rate of isolation of Pseudomonas aeruginosa (PA) and multidrug-resistant PA (MDR-PA) from patients with chronic suppurative otitis media (CSOM) otorrhea and the annual trend of antibiotic-resistance.
Methods
Otorrhea samples were collected aseptically from 1,598 CSOM patients. The rate of bacterial isolation and the results of antibiotic susceptibility testing were evaluated retrospectively.
Results
The PA isolation rate from CSOM otorrhea was 24.4%. Of the 398 isolated strains tested for their susceptibilities to 10 antibiotics, 395 strains showed definitive results. Of these, 183 (46.3%) were susceptible to whole antibiotics and 212 (53.7%) was resistant to more than 1 antibiotics, with the frequency of antibiotics-resistance increasing significantly over time. Although strains susceptible to all antibiotics decreased over time, the rate of isolation of MDR-PA did not change significantly. Resistance to aminoglycosides and quinolones was higher than to other antibiotics and significantly increased over time, whereas resistance to other antibiotics showed no trend.
Conclusion
MDR-PA, assessed using five individual antibiotics and six antibiotic-classes, showed no tendency to increase or decrease over time. This may have been due to increased concern about antibiotic-resistant bacterial strains, leading to improved infection control within hospitals and healthcare centers.
Pseudomonas aeruginosa (PA), an aerobic, nonfermenting Gram-negative rod bacterium, is one of the most troublesome bacteria in hospital-acquired infections. Infections caused by PA can cause various diseases, including otitis externa, osteomyelitis, meningitis, endocarditis, pneumonia, urinary tract infection and septicemia (1).
Although PA is inherently resistant to several antibiotics, it was originally sensitive to the anti-pseudomonal beta-lactam class antibiotics, such as ceftazidime (CAZ), cefepime (CFP), and carbapenem, which were usually used to treat PA infections. More recently, however, bacteria, including PA, have acquired resistances to many antibiotics, giving rise to an increasing number of multidrug-resistant strains resistant to CFP and imipenem (IMP) (2).
Chronic suppurative otitis media (CSOM) is an inflammatory disorder that causes irreversible changes of the mucosa in the middle ear and mastoid cavity. Dysfunction of the Eustachian tubes and bacterial infection are the most important pathogenic factors for CSOM. Owing to the long period of morbidity of CSOM and the repeat occurrences of otorrhea during that period, patients with this condition are often prescribed empiric antibiotics in outpatient clinics without microbiologic evaulation. Bacteria identified in patients with CSOM otorrhea (e.g., Staphylococci and PA) differed from those in patients with acute otitis media (e.g., Streptococcus pneumoniae, Moraxella catarrhalis, and Haemophilus influenzae). One study found that the bacterium isolated most frequently from CSOM otorrhea was Staphylococci, followed by PA (3). If, however, the Staphylococci isolates were divided to methicillin-resistant (MRSA), methicillin-sensitive (MSSA), and coagulase-negative (CNS), then PA was the most commonly isolated bacterium from CSOM otorrhea (3).
To determine the validity of these findings, we assessed the overall rates of isolation of PA and multidrug-resistant PA (MDR-PA) from otorrhea of CSOM. We also analyzed changes over time in the rate of isolation of MDR-PA and in the antibiotic-resistance patterns of PA.
The study population consisted of 1,598 CSOM patients (799 males, 834 females) who visited the Department of Otolaryngology at the outpatient clinics of 6 general hospitals throughout Korea between January 2001 and December 2008. Mean patient age was 45.6 (±17.4) years (range, 1 to 91 years). A total of 1,633 otorrhea samples was collected, with PA isolated from 398 samples from 398 patients (186 males, 212 females). The mean age of these 398 patients was 45.5 (±17.3) years (range, 2 to 91 years). Of these 398 patients, 286 (71.9%) had non-cholesteatomatous CSOM, 106 (26.6%) had cholesteatomatous CSOM, and 6 (1.5%) were unclassified.
At the first visit, the external auditory canal of each patient was cleaned well, and the aural discharge was collected with cotton swabs while using a sterilized otoscope to prevent contact with the external auditory canal. All clinical samples were added to Stuart transport medium and transported to a microbiology test laboratory within 1 hour of collection. The, samples were inoculated into blood agar medium and cultured for 24 hours at 35℃. Bacteria were identified by Gram staining and biochemical tests.
All antibiotic-sensitivity tests followed the guidelines of the Clinical and Laboratory Standards Institute. PA were tested for sensitivity to amikacin (AMK), gentamicin (GM), tobramycin (TOB), CAZ, CFP, piperacillin (PIP), PIP/tazobactam (PITA), IMP, ciprofloxacin (CIP), and levofloxacin (LFX) by the disc diffusion method using Mueller-Hinton agar medium (Difco, Cockeysville, MD, USA) (4).
There is no clear and official definition of MDR. Generally, however, resistance to more than three core antibiotics (i.e., AMK and/or GM, CAZ, CIP, IMP, and PIP-ticarcillin) is classified as MDR (5). In this study, we defined MDR as resistance to at least three of the five core antibiotics (AMK, CAZ, PIP, CIP, and IMP).
In general, antibiotics of the same class act via the same anti-bacterial mechanism. However, differences in absorption, distribution, metabolism and excretion from the human body caused by molecular differences result in pharmacokinetic differences and hence different anti-bacterial potencies. Thus, although AMK, GM, and TOB all belong to the same class of antibiotics (aminoglycosides), AMK is usually the most potent of these three antibiotics. Clinically, bacteria susceptible to AMK are not always susceptible to GM and TOB.
Another approach to enhancing anti-bacterial potency is to add an inhibitory material (a hydrolase) to an antibiotic. For example, PITA is a complex, anti-pseudomonal penicillin (PIP) containing a penicillinase inhibitor (tazobactam). However, alteration of the structure of the bacterial penicillin-binding site can make bacteria resistant not only to PIP but also to PITA. In practice, the definition of MDR as resistance to five core antibiotics may have clinical limitations. In this study, we therefore additionally categorized 10 antibiotics into 5 classes-aminoglycosides (AMK, GM, TOB), cephalosporins (CAZ, CFP), anti-pseudomonal penicillins (PIP, PITA), carbapenem (IMP), and quinolones (CIP, LFX)-and defined modified-MDR (mMDR) as resistance to at least 3 of these 6 classes.
In designations of mMDR, resistance to at least 1 individual antibiotic in each class was defined as resistance to that entire class.
SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The annual changes in overall resistance of PA and MDR-PA isolation rate were analyzed by the linear by linear association method. Statistical significance was defined as a P-value <0.05.
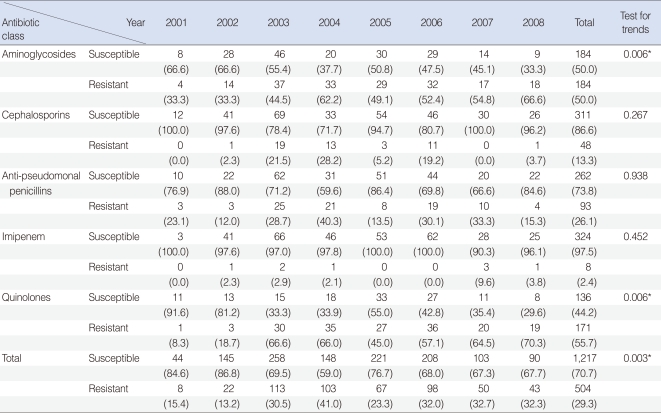
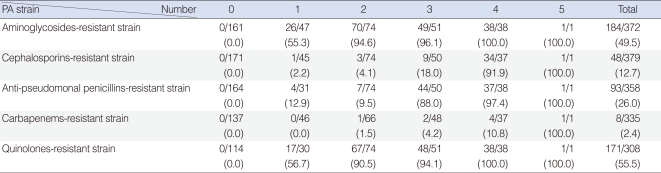
Of the 398 isolated strains tested for sensitivity to 12 antibiotics, 395 showed definitive results, with a total of 3,241 combinations tested. Of these, 183 (46.3%) strains showed susceptibility to all of 10 antibiotics tested. Sixty-two (15.6%) strains were resistant to 3 antibiotics and 38 (9.6%) strains were resistant to 1 antibiotic (Fig. 1). If tested 10 antibiotics were classified into 5 classes, majority of PA strain was susceptible to whole 5 classes antibiotics 183, 46.3%), following resistant to 2 classes (74, 18.7%), 3 classes (51, 12.9%), and 1 class (48, 12.1%) (Fig. 2). Between 2001 and 2008, the rate of antibiotic resistance increased significantly over time, whereas the rate of susceptibility decreased significantly (linear by linear association, P=0.003) (Table 1). The rate of resistance to quinolones (55.7%) was highest, followed by rates of resistance to aminoglycosides (50.0%), anti-pseudomonal penicillins (26.1%) and cephalosporins (13.3%) (Table 1).
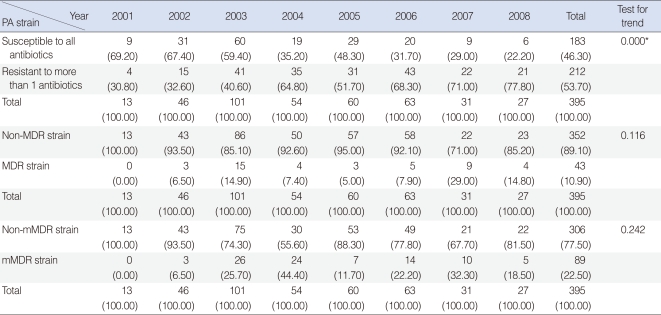
When the strains were classified as susceptible to all antibiotics and resistant to ≥1 antibiotic, we observed a significant decrease in the former over time (Table 2). The rates of isolation of MDR strains, defined as strains resistant to at least three of five antibiotics (AMK, CAZ, PIP, IMP, and CIP), and mMDR strains (i.e., strains resistant to more than three antibiotic-classes), showed no trends over time (Table 2).
Strains resistant to one class of antibiotics tend to be more resistant to aminoglycosides or quinolones than to other classes. In contrast, resistance to cephalosporins or anti-pseudomonal penicillins was less frequent in strains resistant to one class of antibiotics but was high in multi-antibiotic-class resistant strains (Table 3). We found that resistance to aminoglycosides and quinolones significantly increased over time, whereas resistance to the other classes of antibiotics showed no trend (Table 3).
PA can colonize various sites of the human body, including the sputum, cornea, nasal mucosa and wet skin, causing a range of diseases from minor skin infections to fulminant septicemia (6). Some β-lactam antibiotics (e.g., CAZ, CFP, IMP, and meropenem) that show strong anti-pseudomonal activity have been used, alone or in combination with aminoglycosides/quinolones, to treat PA infections (7, 8). The rate of isolation of PA strains resistant to CAZ, CFP, and IMP has increased recently, making it more difficult to select adequate antibiotics. PA can acquire antibiotic resistance through horizontal and vertical transmission (5, 8) and transmission of resistance to several antibiotics (9) can develop into MDR, making treatment more difficult.
Resistance to aminoglycoside can be acquired by several mechanisms including enzyme modification (major), active efflux, low outer membrane permeability, and rarely by target modification. Aminoglycoside modifying enzymes (AMEs) can attach a phosphate, adenylyl or acetyl radical to the antibiotic molecule, and thus they can decrease the binding affinity of the modified antibiotics to the target in the bacterial cell (30s ribosomal unit) (10). Other mechanisms of aminoglycosides can be attributed to a reduced uptake due to the diminished outer membrane permeability (11) and active aminoglycoside efflux due to MexXY proteins operating simultaneously with OprM, as well as with some other outer membrane proteins (12). Methylation of 16S rRNA by a newly recognized group of 16S rRNA methylases is a new mechanism of resistance against aminoglycoside (13).
Two major mechanisms may lead to quinolones resistance to PA. Modification of the primary target (DNA gyrase, also known as topoisomerase II) and secondary target (topoisomerase IV) occurs by point mutations in gyrA/gyrB and parC/pare genes respectively (14). Four efflux systems identified in PA (MexAB-OprM, MexCD-OprJ, MexEF-OprN, and MexXY-OprM) also offer resistance not only to quinolones but also to tetracycline, chloramphenicol, erythromycin, ethidium bromide and acriflavine (15).
Another important mechanism in acquired bacterial resistance to antibiotics is the production of extended-spectrum β-lactamase (ESBL). Most ESBL-producing bacteria show a high degree of resistance to third-generation cephalosporins, including CAZ and CFP. To date, three classes of enzymes have been described, penicillinase-derivatives (class A), metallo-β-lactamases (class B) and oxacillinases (class D). Class A enzymes are encoded on plasmids and may be horizontally transmitted from other enteric Gram-negative bacteria (16, 17). PA strains expressing class A enzymes develop resistance to ureidopenicillins, CAZ, cefpirome, CFP and susceptibility to carboxy- or ureidopenicillin/inhibitor combinations. The class B enzymes hydrolyze IMP and meropenem (18), whereas the Class D enzymes have strong activity against oxacillin and cloxacillin. All of these enzymes, except for OXA-18, are weakly inhibited by clavulanic acid.
The Korean National Survey of Antimicrobial Resistance has reported that 21%, 22%, and 22% of PA strains were resistant to CAZ, CFP, and IMP, respectively (19). Similar results were seen in a report from 12 tertiary hospitals in Korea, in which 21%, 22%, and 26% of PA strains were resistant to CAZ, CFP, and IMP, respectively (20). We found that 3.8%, 20.4%, and 2.4% of the PA strains isolated from patients with CSOM otorrhea were resistant to CAZ, CFP, and IMP, respectively. Differences between our results and those of previous studies may be due to differences in treatment modality and the frequency of antibiotic use between CSOM patients and those with other diseases.
We found that the rate of PA resistance to the 10 antibiotics tested increased significantly over time, whereas the rate of isolation of strains susceptible to all antibiotics decreased significantly. In contrast, the rate of isolation of MDR and mMDR strains showed no tendency to increase. Similar results were observed in the rate of isolation of MRSA from otorrhea (3). This lack of increase may be associated with growing concerns about the development of antibiotic-resistant bacterial strains in hospitals and healthcare centers, prompting more careful infection control.
The non-MDR strains (i.e., those resistant to less than three antibiotics) showed greater resistance to aminoglycosides and quinolones than to other antibiotics. Resistance to cephalosporins and penicillins was particularly low in non-MDR strains, but their rate of isolation increased as the number of drugs to which the strain was resistant increased. Resistance to aminoglycosides and quinolones tended to increase over time, whereas resistance to other antibiotics showed no tendency to increase or decrease. Because PA is inherently resistant, due to its encoding of chromosomal AmpC β-lactamase, a cytoplasmic efflux pump and porin proteins, the strong resistance to aminoglycosides and quinolones of domestic PA strains from patients with otorrhea may be reinforced by a genetic trait or the misuse or overuse of these medications. These strains showed higher resistance to the quinolones CIP (55.5%) and LFX (35.4%) than to other antibiotics (except GM, TOB, SPT). Quinolones are broad-spectrum antibiotics used to treat various PA infections. Moreover, the availability of an oral formulation has increased its use in otolaryngology because most of these patients are treated in outpatient clinics. Quinolones may be prescribed empirically, without culture or antibiotic susceptibility testing, for patients with CSOM otorrhea, which may strengthen resistance to quinolones. This resistance may lead to quinolones no longer being the first-line antibiotic in the treatment of CSOM.
CSOM otorrhea is usually treated using oral medications with otic drops (mainly fluoroquinolones). The absence of oral formulations of CAZ, CFP, and IMP has severely limited the use of these antibiotics in patients with CSOM otorrhea, thus restricting the ability of these bacteria to develop resistance to these antibiotics. IMP is regarded as the final medication for MDR PA infection. Strains resistant to CAZ, CFP, and IMP/meropenem may be difficult to treat due to the absence of adequate antibiotics. This may require treatment with polymyxin (colistin; polymyxin E), which is associated with severe nephrotoxicity and neurotoxicity, severely limiting its usage. As a drug of last resort, however, polymyxin has shown some success. Polymyxin, alone or with a β-lactam antibiotic, was successful in the treatment of patients with septicemia, pneumonia, and urinary tract infections (21, 22). In otolaryngology, fluoroquinolones are usually prescribed as otic drops even if the isolated strains are resistant to quinolones because the concentration of antibiotic in otic drops in the middle ear and mastoid cavities is high enough to overcome the minimal inhibitory concentrations of resistant strains. Moreover, treatment outcomes are quite good. Surgery also plays an important role in the treatment of CSOM. Many patients can be cured by otic drops and surgery, thus reducing the use of last-resort drugs such as IMP. This may explain, at least in part, why resistance to IMP in bacteria from CSOM otorrhea is lower than in other types of bacterial infection (19, 20).
The rate of isolation of PA from CSOM otorrhea was 24.4% and the total resistance rate was 28.2%. MDR strains classified by resistance to five individual antibiotics and mMDR strains classified by resistance to six classes of antibiotics showed no tendency to increase or decrease over time. However, the resistance rates and patterns of resistance to each antibiotic were quite different. Because all analyzed PA strains were obtained from clinical samples taken at outpatient clinics of primary care centers or tertiary teaching hospitals, these strains likely represent the general characteristics of PA in CSOM otorrhea in Korea. Although we assessed the rates of isolation of PA and MDR-PA from otorrhea, we did not examine the antibiotics actually used, the treatment results, or the antibiotic-resistance patterns relative to the use of previous antibiotics. Further studies are required to determine the nature of MDR-PA infections.
References
1. Bergogne-Berezin E. Cohen J, Powderly WG, editors. Pseudomonas and miscellaneous gram-negative bacilli. Infectious diseases. 2004. 2nd ed. Philadelphia, PA: Mosby;p. 2203–2226.
2. Song W, Woo HJ, Kim JS, Lee KM. In vitro activity of beta-lactams in combination with other antimicrobial agents against resistant strains of Pseudomonas aeruginosa. Int J Antimicrob Agents. 2003; 1. 21(1):8–12. PMID: 12507832.
3. Park DC, Lee SK, Cha CI, Lee SO, Lee MS, Yeo SG. Antimicrobial resistance of Staphylococcus from otorrhea in chronic suppurative otitis media and comparison with results of all isolated Staphylococci. Eur J Clin Microbiol Infect Dis. 2008; 7. 27(7):571–577. PMID: 18299908.

4. Wikler MA. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: sixteenth informational supplement. 2006. Wayne, PA: Clinical and Laboratory Standards Institute.
5. Livermore DM. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin Infect Dis. 2002; 3. 01. 34(5):634–640. PMID: 11823954.

6. Pollack M. Mandell GL, Bennett JE, Dolin R, editors. Pseudomonas aeruginosa. Mandell, Douglas and Bennett's principles and practice of infectious diseases. 2000. 5th ed. Edinburgh, UK: Churchill Livingstone;p. 2310–2327.
7. Jones RN, Kirby JT, Beach ML, Biedenbach DJ, Pfaller MA. Geographic variations in activity of broad-spectrum beta-lactams against Pseudomonas aeruginosa: summary of the worldwide SENTRY Antimicrobial Surveillance Program (1997-2000). Diagn Microbiol Infect Dis. 2002; 7. 43(3):239–243. PMID: 12106958.
8. Walsh TR, Toleman MA, Hryniewicz W, Bennett PM, Jones RN. Evolution of an integron carrying blaVIM-2 in Eastern Europe: report from the SENTRY Antimicrobial Surveillance Program. J Antimicrob Chemother. 2003; 7. 52(1):116–119. PMID: 12805257.

9. Carmeli Y, Troillet N, Eliopoulos GM, Samore MH. Emergence of antibiotic-resistant Pseudomonas aeruginosa: comparison of risks associated with different antipseudomonal agents. Antimicrob Agents Chemother. 1999; 6. 43(6):1379–1382. PMID: 10348756.
10. Llano-Sotelo B, Azucena EF Jr, Kotra LP, Mobashery S, Chow CS. Aminoglycosides modified by resistance enzymes display diminished binding to the bacterial ribosomal aminoacyl-tRNA site. Chem Biol. 2002; 4. 9(4):455–463. PMID: 11983334.

11. MacLeod DL, Nelson LE, Shawar RM, Lin BB, Lockwood LG, Dirk JE, et al. Aminoglycoside-resistance mechanisms for cystic fibrosis Pseudomonas aeruginosa isolates are unchanged by long-term, intermittent, inhaled tobramycin treatment. J Infect Dis. 2000; 3. 181(3):1180–1184. PMID: 10720551.
12. Jo JT, Brinkman FS, Hancock RE. Aminoglycoside efflux in Pseudomonas aeruginosa: involvement of novel outer membrane proteins. Antimicrob Agents Chemother. 2003; 3. 47(3):1101–1111. PMID: 12604548.
13. Doi Y, Arakawa Y. 16S ribosomal RNA methylation: emerging resistance mechanism against aminoglycosides. Clin Infect Dis. 2007; 7. 01. 45(1):88–94. PMID: 17554708.

14. Hooper DC. Emerging mechanisms of fluoroquinolone resistance. Emerg Infect Dis. 2001; Mar-Apr. 7(2):337–341. PMID: 11294736.

15. Masuda N, Sakagawa E, Ohya S, Gotoh N, Tsujimoto H, Nishino T. Substrate specificities of MexAB-OprM, MexCD-OprJ, and MexXY-oprM efflux pumps in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2000; 12. 44(12):3322–3327. PMID: 11083635.
16. Vahaboglu H, Hall LM, Mulazimoglu L, Dodanli S, Yildirim I, Livermore DM. Resistance to extended-spectrum cephalosporins, caused by PER-1 beta-lactamase, in Salmonella typhimurium from Istanbul, Turkey. J Med Microbiol. 1995; 10. 43(4):294–299. PMID: 7562992.
17. Mugnier P, Dubrous P, Casin I, Arlet G, Collatz E. A TEM-derived extended-spectrum beta-lactamase in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1996; 11. 40(11):2488–2493. PMID: 8913451.

18. Watanabe M, Iyobe S, Inoue M, Mitsuhashi S. Transferable imipenem resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1991; 1. 35(1):147–151. PMID: 1901695.

19. Lee K, Kim YA, Park YJ, Lee HS, Kim MY, Kim EC, et al. Increasing prevalence of vancomycin-resistant enterococci, and cefoxitin-, imipenem- and fluoroquinolone-resistant gram-negative bacilli: a KONSAR study in 2002. Yonsei Med J. 2004; 8. 31. 45(4):598–608. PMID: 15344199.

20. Lee H, Yong D, Lee K, Hong SG, Kim EC, Jeong SH, et al. Antimicrobial resistance of clinically important bacteria isolated from 12 hospitals in Korea in 2004. Korean J Clin Microbiol. 2005; 4. 8(1):66–73.
21. Falagas ME, Kasiakou SK. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis. 2005; 5. 01. 40(9):1333–1341. PMID: 15825037.

22. Falagas ME, Bliziotis IA, Kasiakou SK, Samonis G, Athanassopoulou P, Michalopoulos A. Outcome of infections due to pandrug-resistant (PDR) Gram-negative bacteria. BMC Infect Dis. 2005; 4. 08. 5:24. PMID: 15819983.

Fig. 1
Frequency of isolated Pseudomonas aeruginosa strains from otorrhea, as determined by antibiotic-susceptibility test.
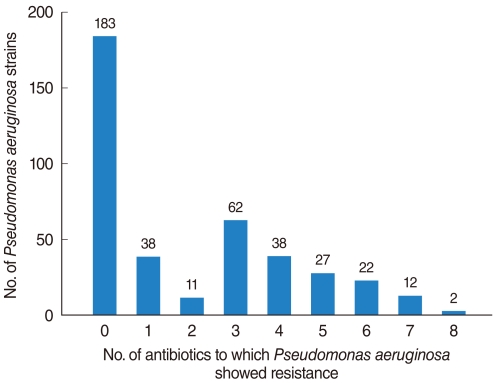
Fig. 2
Frequency of isolated Pseudomonas aeruginosa strains from otorrhea, as determined by resistance to six classes of antibiotics.
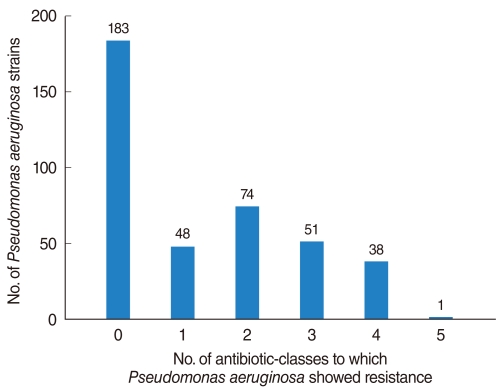
Table 1
Annual isolation rate of Pseudomonas aeruginosa strains susceptible and resistant to each antibiotic class




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download