Abstract
Objectives
Apoptosis may play an important role in the mechanism underlying the GJB2 gene conditional knockout (cCx26) mice cochlear cell death. The objective of this study was to explore the the damage mode of the outer hair cells (OHCs) and its real time point of apoptosis and provide information to further explore the role of apoptosis in the happening of hearing loss in cCx26 mice.
Methods
Cochleae from mice at various developmental stages (P8, P12, and P21) were dissected out and first used to be observed under the scanning electron microscope (SEM). Basilar membranes from mice at P8, P14, P18, and P21 were stained by fluorescein isothiocyanate-conjugated phalloidin and propidium iodide (PI) and examined under confocal microscope.
The GJB2 gene located on chromosome 13q12 encodes connexin26 (Cx26), a transmembrane protein involved in the cell-cell attachment of almost all tissues. Cx26 is one of the most prevalent connexins in the cochlea. Its homozygous and heterozygous gap junctions play an important role in the nutrition and metabolism of the inner ear. Previous studies have shown that GJB2 gene mutation is the most common cause of non-syndromic hearing impairment (NSHI) in humans. To date, more than 120 GJB2 mutations have been found responsible for NSHI, most of which are loss-of-function mutations [1].
Conditional knockout mice, after the embryonic lethality of Cx26 knockout mice [2], are alternative animal models for studying the mechanism of NSHI. To date, three kinds of Cx26 conditional knockout mice (cCx26) have been reported: Cx26loxP/loxP OtogCre [3], Cx26loxP/loxP Pax2Cre, and Cx26loxP/loxP foxg1Cre [4]. The differences in the induction mechanism among the three mice are based on the different promoters driving the Cre gene, which results in different time-specific cCx26 mice. The three mice revealed changes caused by cCx26 depletion in the inner ear were mostly in common. The inner ear is well developed at birth. Cell death first occurs in the supporting cells during P8 [4] or P14 [3]. In addition, the sensory hair cells die soon after the onset of hearing at around P14. Activated caspase 3 has been detected in cCx26 mice cochlea, which suggests that apoptosis might be the mechanism underlying the cCx26 mice cochlear cell death [3]. Most reports focused on the death of supporting cells damage rather than the destruction of hair cells following GJB2 gene knockout. The gross surface morphology of cochlea has not been reported. In order to know the real role of apoptosis in the happening of the hearing loss in cCx26 mice, the definite damage time of the outer hair cells (OHCs) must be set. The present study observed the surface morphology of the cochlea in cCx26 mice under scanning electron microscope (SEM) and confocal microscope. The destruction manner and progression of OHC apoptosis were studied. The destruction manner of OHC following GJB2 gene knockout may be via apoptosis. And typical apoptotic morphology was found in the OHCs of the cCx26 mice at P18.
The 309th Hospital Animal Care and Use Committee approved the use of animal models in the present study. Cx26loxP/loxP Pax2Cre mice were generated by crossbreeding Cx26loxP/loxP and Pax2Cre mice. Both transgenic mice were obtained from the laboratory of Dr. Xi Lin. The genotype of the mice was defined via polymerase chain reaction (PCR). The loxP sequences were detected using the following primer pairs: forward 5'-ACAGAAATGTGTTGGTGATGG-3' and reverse 5'-CTTTCCAATGCTGGTGGAGTG-3'. The Cx26loxP/loxP and the wild-type mice generated 322 and 288 bp band, respectively. The larger band was caused by the insertion of the 34 bp loxP sequence around the GJB2 gene. The presence of CreER was detected using the following primer pair: forward 5'-AGCTAAACATGCTTCATCGTCGGTC-3' and reverse 5'-TATCCAGGTTACGGATATAGTTCATG-3'. This resulted in a 700 bp PCR product. The PCR protocol was as follows: 95℃ for 5 minutes, 35 cycles of 95℃ for 1 minute, 55℃ for 1 minute, and 72℃ for 1 minute.
Mice at various developmental stages (P8, P12, and P21) were used. They were anesthetized with intraperitoneal injections of chloral hydrate at 400 mg/kg (4% solution in phosphate-buffered saline [PBS], pH 7.4). The animals were decapitated; their cochleae were removed and fixed in glutaraldehyde (2.5% in phosphate buffer, 0.1 M) for 6 hours. The round window and apex of the cochlea were opened to provide access to the fixative. Samples were decalcified using 10% EDTA at 4℃ for 72 hours. The specimens were then rinsed with 0.1 M phosphate buffer (pH 7.4) and fixed again in osmium tetroxide (1% dissolved in 0.2 M sodium cacodylate buffer) for 2 hours at room temperature. Subsequently, they were perfused and were washed three times (15 minutes each) in phosphate buffer. Dehydration was carried out up to 70% alcohol (15 minutes each). The samples were dissected to remove the bony wall of the cochlea and expose the organ of Corti. The specimens were then prepared for SEM (S4800; Hitachi, Tokyo, Japan) after critical point drying and coating with gold-palladium.
Various developmental stages mice (P8, P14, P18, and P21) were used. The animals older than P8 were fixed via cardiac perfusion in 4% paraformaldehyde dissolved in PBS (pH 7.4). The dissected cochleae were fixed again in paraformaldehyde (4%) at 4℃ overnight and then decalcified in 10% EDTA solution for 72 hours. The organ of Corti was microdissected, rinsed in PBS, and immersed into 0.25% Triton X-100 for 5 minutes. It was then placed in fluorescein isothiocyanate (FITC)-conjugated phalloidin (Enzo, 1:200) in PBS for 30 minutes to label the stereocilium bundles and hair cell cuticular plates. The specimens were rinsed in PBS and then stained with 10 µg/mL propidium iodide (PI) for 10 minutes to label the nuclei selectively. The samples were subsequently rinsed with PBS, mounted on glass slides using Fluoromount-G™ (Southern Biotech, Birmingham, AL, USA), and examined under an LSM 510 confocal microscope (Zeiss, Jena, Germany). OHCs nuclei stained by PI showing shrunken, condensed, fragmented and their cuticular stained by FITC phollidin showing abnormal were considered apoptosis.
The approximate time point of death of the hair cell was first identified via SEM between P12 and P21. SEM observations were conducted on mice at P8, P12, and P21. The cochleae were observed throughout their length. The surface morphology of the basilar membrane on P8 in cCx26 mice developed normally without obvious differences from the wild type mice (Fig. 1A1-C1). In the basal turn, one row of inner hair cells (IHCs) and three rows of OHCs were observed. The stereocilia on the three rows of OHC were arranged in a W-shape whereas those on the IHCs formed a gently curving arc similar to those in normal ears. In the middle turn, an extra OHC row was observed. Kinocilia were seen on top of the OHCs and IHCs. In the apical turn, the IHCs did not fully develop and extra IHCs could be seen. Most of the OHCs ranked irregularly with kinocilia. The OHCs and IHCs were surrounded by microvilli.
The surface morphology of the organ of Corti seemed still normal at the P12 stage (Fig. 1A2-C2). However, extensive damage to the stereocilia and cuticular plate of OHC in the basal and middle turn of the cochlea was observed at the P21 stage (Fig. 2). The stereocilia and cuticular plate were absent or severely damaged on the OHC (Fig. 2A1, B1). Sporadic deletion of OHC was also observed (Fig. 2B1, B2). The damage was severe in the basal (Fig. 2A1-C1) turn and the middle turn (Fig. 2A2-C2) at this stage. The morphology of the apical turn (Fig. 2A3-C3) was intact with only irregular OHC stereocilia. In contrast to the massive damage to the OHCs, the IHCs developed fully. The IHCs showed no obvious pathologic changes regardless of their locations (Fig. 2C1-C3). These results indicate that the OHCs of the cochlea were damaged between P12 and P21. In addition, this damage is much more severe in the middle and basal turn of the cochlea than that in the apical turn.
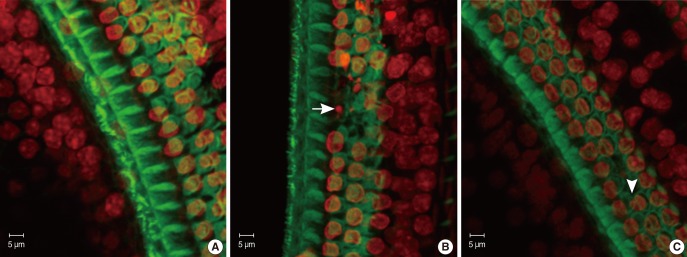
The specimens were stained with PI to label the nuclei, counterstained with FITC-conjugated phalloidin, and evaluated under a confocal microscope to visualize the hair-cell nuclei and cuticular plates. Fig. 3 showed the surface preparation of the organ of Corti in wild type animals at P8 (Fig. 3B1), P14 (Fig. 3B2), and P21 (Fig. 3B3). The PI-labeled OHC nuclei were large, round, evenly stained, and arranged in three orderly rows. Phalloidin staining revealed a pale green cuticular plate and the intense, thin green ring at the boundary between the OHC and adjacent supporting cells. The appearance of basilar membrane in cCx26 mice (Fig. 3A1) showed normal morphologies as that of the wild-type mice did at the P8 stage. The cuticular plates during the P14 stage were a little loose on the apical turn but showed no obvious shrinkage and damage. The OHC nuclei in the P14 cochlea were less regularly arranged than those of the wild type. Some of their nuclei were deeply stained with PI and unevenly quasi-circular (Fig. 3A2). Obvious changes in surface morphology were observed during the P18 stage. The OHC nuclei in the middle and basal turns of the cCx26 cochlea were shrunken, condensed, fragmented (arrow), and in some cases, completely missing (arrow head) sporadically (Fig. 4). The FITC phalloidin labeling of the middle and basal turns of the cuticular plate around the OHCs appeared fuzzy and irregular (Fig. 4B, C), which may indicate cuticular plate damage. The aforementioned changes were more severe in the middle turn (Fig. 4B) than in the basal turn (Fig. 4C). In contrast to the basal and middle turns, the morphologic changes in the apical turn only showed different shapes in the stained OHC nuclei without obvious loss or damage (Fig. 4A). These morphological changes in the nuclei clearly indicate that hair cells are undergoing apoptosis in the cCx26 mice at the P18 stage. The cuticular plate around the OHC was fuzzy at this stage. Thus, the supporting cell surrounding the OHC, such as Dieter's cells, might begin to be damaged sporadically. More severe damage on the organ of Corti in the cCx26 mice was observed during the P21 stage. Loss of OHCs was detected in the whole length of the basilar membrane. The middle turn revealed segmental breakage of the basilar membrane, which indicates the deletion of OHCs and also the supporting cells surrounding them (Fig. 3A3).
In the present study, the loss of OHCs of cCx26 knockout mice was first set between P12 and P21 under SEM. Whole mount stained by phalloidin and PI staining were used to observe detailed surface morphologic changes of the basilar membrane. The destruction of hair cells following GJB2 gene knockout may be via apoptosis. Obvious apoptotic appearance of the OHC surface morphology was observed at P18, including sporadic shrinkage, condensation, fragmentation, and complete loss. During P21, severe damage was observed on the OHC stereocilia and cuticular plate in the basilar membrane, especially in the middle turn of the cochlea.
Apoptosis is endogenous programmed cell death. It requires active participation of the cell itself, leaving a dead cell with intact plasma membrane and organelles. It also causes cellular volume reduction and chromatin condensation that proceeds later to nuclear fragmentation [5]. Reports estimate that either too little or too much apoptosis contribute to a significant number of medical illnesses, including oncogenesis [6].
To date, three kinds of cCx26 mice have been reported. In spite of the technical approaches used in different studies to generate mutant mice, these methods share some common phenomena. No gross developmental defects in the cochlear morphology of newborn mice are detected in all three cCx26 mice, suggesting that the Cx26 does not play an essential role in cochlear development before birth. Light microscope observations reveal that none of the mouse models displays obvious endolymphatic hydrops and stria vascularis degeneration. Hair cell and supporting cell loss after the hearing onset are observed in all mouse models. However, the IHC in all the three kinds of mouse models were morphologically intact until the adult stage [1]. Cx26 was conditionally knocked out at different specific times because of the different promoters driving the Cre gene. The phenotype generated by the different technical approaches resulted in diverse cell loss patterns. In the Cx26loxP/loxP OtogCre mice, the Cx26OtogCre cochlea was indistinguishable from that of the wild type mice up to P14 [3]. The organ of Corti cells died in the following sequence: supporting cells surrounding the IHC, supporting cells around OHC, OHC, and all other organ of Corti cells except IHC and spiral ganglions. Approximately 30 dB SPL elevation in the frequencies was tested in the click auditory brainstem response (ABR). In addition, caspase 3 was detected in the organ of Corti during P17, indicating that apoptosis is the mechanism underlying the cell death in cCx26 mice [3]. However, in the Cx26loxP/loxP Pax2Cre mice, the tunnel of Corti and Neul's space had never opened since P9. The cell death in organ of Corti was first observed in Claudius cells, followed by OHC and spiral ganglions. The hearing loss was more severe in the Cx26loxP/loxP Pax2Cre mice with 70-90 dB SPL elevation in almost all the frequencies tested by click ABR [4]. However, all of the previous experiments focused more on the gross cell death in the organ of Corti and less on the real progress of OHC damage. In order to know the real role of apoptosis in the happening of the hearing loss in cCx26 mice, the definite damage time of the OHCs must be set.
Here SEM and fluorescent confocal microscope were used for the surface morphological observations of the cCx26 mice cochlea to reveal the progression of OHCs damage. PI is a fluorescent dye that binds to DNA in dead cells. It can be used to analyze intact apoptotic and dead cells still with cell construct quantitatively. In the fixed cochlea, four kinds of nuclei can be observed in the PI-stained basilar membrane: normal, apoptotic, necrotic, and absent. They can be distinguished through morphologic observation. Nuclear condensation and fragmentation are anatomical hallmarks of apoptosis [7], whereas nuclear swelling is typical features of necrosis [8]. Apoptosis in the OHCs having either condensed or fragmented nuclei was confirmed by examination of caspase 3 activity [9, 10]. Cochlea post-exposure to intensive noise double analyzed by both TUNEL assay and PI staining revealed TUNEL was positive in OHCs cells with condensed and fragmented nuclei. Therefore PI staining is a kind of method which is easy and reliable to observe apoptosis cells in cochlea whole mount staining [11]. Moreover, caspase 3 was detected in the organ of Corti during P17 and from P16 OHCs were labeled positive by TUNEL in Cx26loxP/loxP OtogCre, mice, indicating that apoptosis is the mechanism underlying the cell death in cCx26 mice [3]. Moreover, palloidin preferentially labels filamentous actin in the hair cell stereocilia and cuticular plate. The absence of stereocilia and cuticular plates suggests cell death and melting. Therefore, the basilar membrane double stained with FITC-conjugated phalloidin and PI reveals not only the hair cells experiencing early stage of apoptosis, but also its relationship with stereocilia and cuticular plate in the different stages of apoptosis.
In the present study, OHC deletion was initially set from P12 to P21 via SEM. Whole mount phalloidin and PI staining was employed to observe morphological changes and reveal the definite apoptosis time of OHC. The obvious apoptotic appearance of the OHCs was observed during P18, including sporadic nuclear shrinkage, condensation, fragmentation, and complete absence. Severe damage to the stereocilia and cuticular plate of the OHCs in the basilar membrane, especially in the middle turn of the cochlea, were observed during the P21 stage. The loose cuticular plate and intact OHCs during P14 suggest that the supporting cells may have been influenced by the Cx26 knockout before typical apoptosis of OHCs appeared. This is because cuticular plate around the OHCs is mostly composed of the tight junctions between the OHCs and their supporting cells. As the damage progressed in P18, the cuticular plate around the abnormal OHC appeared vague under FITC-phalloidin staining. This demonstrates that the Dieter's cells might begin to disintegrate at this stage. With continued development, distinct rupture of the basilar membrane occurred during P21, especially in the middle turns. Transmission electron microscopic analyses of the organ of Corti in the Cx26OtogCre mice at the P30 stage showed that the cuticular plate disruption was caused by damage to the function of the Dieter's cells and non-adherence to the OHCs. The cuticular plate damage is likely due to the degeneration of the supporting cells of the hair cells because similar to that often observed after aminoglycoside treatment or noise exposure, when only OHCs died, their supporting cells are able to preserve the integrity of the cuticular plate [3]. Considering that Cx26 is extensively expressed in the supporting cells, its deletion in the cCx26 knockout mice may trap the OHCs in an isolated environment and trigger their apoptosis.
According the traveling theory of the basilar membrane, different parts of the cochlea receive sound at different frequencies. The middle turn of the cochlear produces maximal movement to the sound at frequencies ranging from 1,000 Hz to 4,000 Hz, in which most of the natural frequencies exist. Therefore, the middle turn may be the most active part in the cochlea and it may be vulnerable to different kinds of stimulation. The progression of the cochlear lesion following exposure to traumatic noise revealed that the lesion started in the middle turn, the section of the cochlear that receives maximal stimulation. From this center, the lesion expands toward both the apical and basal turns [9]. In the present study, more severe damage to the middle turn of the basilar membrane was observed whether in P18 or in P21, which is also mentioned in a previous study on the section of the cochlea in cCx26 knockout mice [4]. Given that the inner ear cells begin to die after the hearing starts in Cx26 knockout mice, the heavy cell damage in the middle turn may be due to the frequencies to which it is wherein receptive. The middle turn is the main part of the cochlea that receives stimulation because mice start hearing at 1,000-4,000 Hz. The deletion of the Cx26 caused the cells in the organ of Corti to be trapped in an isolated environment without a nutritional supply and vulnerable to stimulation. This may result in more severe cell damage in the middle turn than the apical and basal turns of the cochlea.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (No. 30700936), Scientific Research Foundation for Returned Scholars from Chinese PLA during the 11th Five-Year Plan period (No. 06H048) and Scientific Research Foundation from Chinese PLA during the 12th Five-Year Plan period (11MS013) to Yanping Zhang.
References
1. Hoang Dinh E, Ahmad S, Chang Q, Tang W, Stong B, Lin X. Diverse deafness mechanisms of connexin mutations revealed by studies using in vitro approaches and mouse models. Brain Res. 2009; 6. 1277:52–69. PMID: 19230829.

2. Gabriel HD, Jung D, Butzler C, Temme A, Traub O, Winterhager E, et al. Transplacental uptake of glucose is decreased in embryonic lethal connexin26-deficient mice. J Cell Biol. 1998; 3. 140(6):1453–1461. PMID: 9508777.

3. Cohen-Salmon M, Ott T, Michel V, Hardelin JP, Perfettini I, Eybalin M, et al. Targeted ablation of connexin26 in the inner ear epithelial gap junction network causes hearing impairment and cell death. Curr Biol. 2002; 7. 12(13):1106–1111. PMID: 12121617.

4. Wang Y, Chang Q, Tang W, Sun Y, Zhou B, Li H, et al. Targeted connexin26 ablation arrests postnatal development of the organ of Corti. Biochem Biophys Res Commun. 2009; 7. 385(1):33–37. PMID: 19433060.

5. Tadros SF, D'Souza M, Zhu X, Frisina RD. Apoptosis-related genes change their expression with age and hearing loss in the mouse cochlea. Apoptosis. 2008; 11. 13(11):1303–1321. PMID: 18839313.

6. Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007; 6. 35(4):495–516. PMID: 17562483.

7. Ding D, Jiang H, Salvi RJ. Mechanisms of rapid sensory hair-cell death following co-administration of gentamicin and ethacrynic acid. Hear Res. 2010; 1. 259(1-2):16–23. PMID: 19715747.

8. Majno G, Joris I. Apoptosis, oncosis, and necrosis: an overview of cell death. Am J Pathol. 1995; 1. 146(1):3–15. PMID: 7856735.
9. Hu BH, Henderson D, Nicotera TM. Involvement of apoptosis in progression of cochlear lesion following exposure to intense noise. Hear Res. 2002; 4. 166(1-2):62–71. PMID: 12062759.

10. Hu BH, Henderson D, Yang WP. The impact of mitochondrial energetic dysfunction on apoptosis in outer hair cells of the cochlea following exposure to intense noise. Hear Res. 2008; 2. 236(1-2):11–21. PMID: 18082984.

11. Yang WP, Hu BH. Comparison of three methods for detecting the death modes of hair cells in the cochlea. Chin J Otol. 2004; 2(4):301–304.
Fig. 1
Scanning electron microscope (SEM) analysis of cochlea in cCx26 knockout mice in P8 (A1-C1), P12 (A2-C2). There were no obvious differences in surface morphology under SEM between knockout mice and wild type one in P8 and P12.

Fig. 2
Scanning electron microscope analysis of cochlea in cCx26 knockout mice in P21. The stereocilia and cuticular plate were absent or severely damaged on the outer hair cell (OHC; A1, B1). Sporadic deletion of OHC was also observed (B1, B2). The damage decreased from the basal (A1-C1) to the middle turn (A2-C2) at this stage. The morphology of the apical turn (A3-C3) was intact with only irregular OHC stereocilia. In contrast to the massive damage to the OHCs, the inner hair cells showed no obvious pathologic changes regardless of their locations (C1-C3).
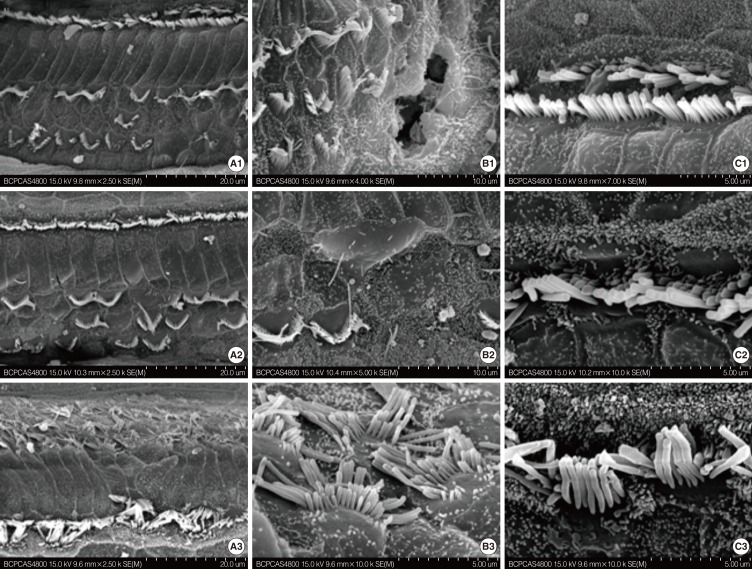
Fig. 3
Confocal microscope analysis of cochlea whole mount in wild type or cCx26 knockout mice stained by fluorescein isothiocyanate-pholloidin and propidium iodide on P8, P14, and P21. The surface morphology of middle turn of cochlea in P8 knockout mice (A1) showed no obvious differences with that of wild type one (B1). In P14, the cuticular plate revealed slightly loose and the nuclei of outer hair cell (OHC) became irregular in apical turn in cochlea of cCx26 knockout mice (A2) compared with that of wild type one (B2). Severe damage was presented on P21 in cochlea of cCx26 knockout mice (A3) compared with those of wild type one (B3). Loose cuticular plate and sporadic deletion of OHC was found in the apical turn. And segmental deletion of the basilar membrane and OHC was demonstrated in the middle turn (A3).
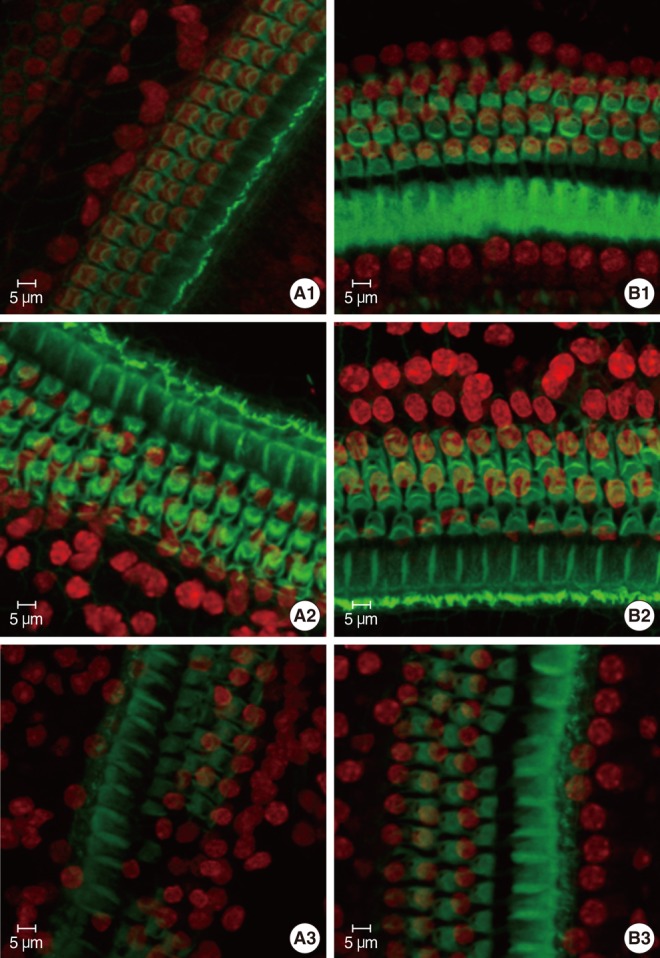
Fig. 4
Confocal microscope analysis of cochlea whole mount in wild type or cCx26 knockout mice stained by fluorescein isothiocyanate (FITC)-pholloidin and propidium iodide. Significant changes were observed in cochlea of cCx26 knockout mice on P18 (A-C). Outer hair cell (OHC) nuclei in the middle (B) and basal turn (C) of the cCx26 cochlea were shrunken, condensed, fragmented (arrow) and in some cases completely missing (arrow head). The FITC phalloidin labeling of the middle and basal turn of the cuticulate plate around the OHC above appeared fuzzy and irregularly, which may indicate the damage of the cuticular plate.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download