INTRODUCTION
METHODS
In vitro experiments
Cell culture
Cell viability assay
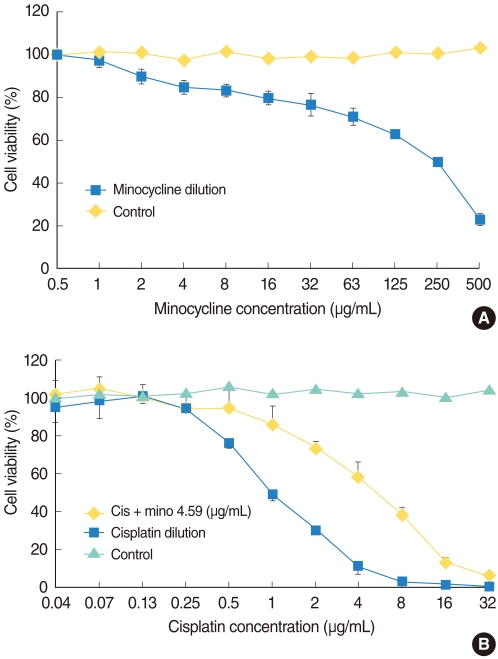 | Fig. 1(A) Cell survival curve of House Ear Institute-Organ of Corti 1 (HEI-OC1) cells cultured with minocycline. Minocyline (mino) is cytotoxic at high, but not at low, concentrations. (B) Cell survival curve of HEI-OC1 cells cultured with cisplatin alone and with cisplatin (cis) after minocycline pretreatment. More cells survived in the 10 µM minocycline pretreatment condition compared with the cisplatin alone condition. |
Western blot analysis
Mitochondrial fractionation
In vivo experiments
Animals
Auditory tests
Scanning electron microscopy
Statistical analysis
RESULTS
In vitro experiments
Cell viability assay
Western blot analysis
Effect of minocycline on the caspase-dependent pathway: Bcl-2, and caspase 3
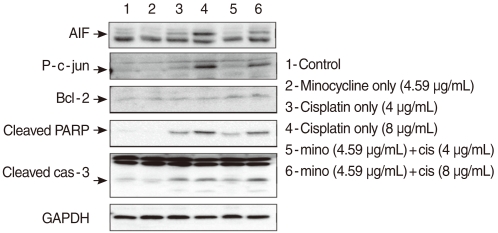 | Fig. 2Cells pretreated with 10 µM minocycline and cultured in 4 µg/mL or 8 µg/mL cisplatin were analyzed using the Western blotting technique with antibodies targeting Bcl-2, p-JUN, cleaved caspase-3, cleaved polymerase (PARP), and AIF. Bcl-2 expression was elevated after the pretreatment with minocycline. Minocycline pretreatment decreased cisplatin-induced cleaved caspase 3 activity at the 4 µg/mL, but not the 8 µg/mL dose. The expression of p-JUN, cleaved PARP, and apoptosis-inducing factor (AIF) increased by cisplatin treatment, and suppressed by minocycline pretreatment. |
Effect of minocycline on the caspase-independent pathway: JNK, PARP, and AIP
In vivo experiments
Auditory brainstem response
 | Fig. 3The distribution (A) and mean amount (B) of hearing threshold shift in each group. The shift was greater in group 2 (white circle) than it was in group 1 (black circle). The threshold shift in the 16 Hz tone burst significantly different between the two groups. The threshold shift was less than 10 dB in two animals pretreated with minocycline. |
SEM evaluation
 | Fig. 4Scanning electron micrographs of the basal turn of the organ of Corti. (A) In a hearing-preserved animal pretreated with minocycline followed by cisplatin treatment, the outer hair cells are well preserved with minimal loss. (B) In an animal treated with cisplatin alone, catastrophic destruction occurred in the outer hair cells with fusion of cilia and bulla formation. The inner hair cells are relatively well preserved compared with the outer hair cells in both groups. |
DISCUSSION
 | Fig. 5The cisplatin-induced apoptosis pathway in House Ear Institute-Organ of Corti 1 (HEI-OC1) cells and the proposed mechanism mediating the protective effect of minocycline. The black arrow (→) indicates activation, and the blocked arrow head (→|) indicates inhibition. Our data show that minocycline activates Bcl-2 and decreases cleaved caspase-3, poly (ADP-ribose) polymerase (PARP), apoptosis-inducing factor (AIF) activity. These findings and those of previous studies suggest that minocycline activates Bcl-2 and inhibits downstream caspase-3 in the caspase-dependent pathway, and suppresses PARP-1 and downstream AIF in the caspase-independent pathway. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download