Abstract
Objectives
Alpha1-antitrypsin (AAT) is the main inhibitor of human neutrophil elastase, and plays a role in counteracting the tissue damage caused by elastase in local inflammatory conditions. The study evaluated the involvement of AAT in nasal allergic inflammation.
Methods
Forty subjects with mono-sensitization to Dermatophagoides pteronyssinus (Dpt) were enrolled. Twenty allergic rhinitis patients frequently complained of nasal symptoms such as rhinorrhea, stuffiness, sneezing, and showed positive responses to the nasal provocation test (NPT) with Dpt (Group I). The other 20 asymptomatic patients showed sensitization to Dpt but negative NPT (Group II). The levels of AAT, eosinophil cationic protein (ECP), and Dpt-specific IgA antibodies were measured in the nasal lavage fluids (NLFs), collected at baseline, 10 minutes, 30 minutes, 3 hours, and 6 hours after the NPT. Nasal mucosa AAT expression was evaluated with immunohistochemical staining from Group I and Group II.
Results
At baseline, only the Dpt-specific IgA level was significantly increased in the NLFs of Group I compared with Group II, while ECP and AAT levels were not significantly different between two groups. After Dpt provocation, AAT, ECP, and Dpt-specific IgA levels were significantly increased in the NLFs of Group I during the early and late responses. The protein expression level of AAT was mostly found in the infiltrating inflammatory cells of the nasal mucosa, which was significantly increased in Group I compared to Group II.
Allergic rhinitis (AR) is characterized by allergen-induced nasal inflammation. An IgE-dependent release of mediators and the recruitment of leukocytes to the nasal mucosa following allergen challenge are widely acknowledged to be critical to the development the main symptoms of AR, such as nasal congestion, watery rhinorrhea, sneezing, and itching sensation. Since the nasal mucosa is the first region of the respiratory tract in contact with an allergen, the nasal lavage fluids (NLFs) produced following allergen challenge are rich in inflammatory cells and mediators (1).
Both eosinophils and neutrophils are present during airway inflammation. Both eosinophil and neutrophil granules contain potent mediators that may cause considerable damage to the surrounding cells and tissues. Among these mediators, preformed serine proteases are contained in the cytoplasm granules of neutrophils; of these, elastase is the most important (2). The main inhibitor of neutrophil elastase is α1-antitrypsin (AAT), which is crucial in the homeostasis of connective tissue turnover during inflammation (3). AAT is synthesized primarily by hepatocytes and, to a lesser extent, by monocytes, macrophages, epithelial cells and trophoblasts (4-6). In addition, AAT is also synthesized and stored in the specific granules of activated neutrophils and eosinophils, which play a role in counteracting the tissue damage caused by elastase in local inflammatory conditions (7, 8). Moreover, an increment of AAT level was observed in allergic inflammation (9, 10). We hypothesized that AAT would be secreted from activated eosinophils in allergic inflammation, because activated eosinophils are the hallmark of allergic inflammation and might be a potent source of AAT.
Previously, we tried to elucidate the immunologic materials involving in allergen-induced nasal inflammation, and found that allergen-specific IgA or vascular endothelial growth factor (VEGF) is increased in the NLFs of symptomatic AR patients after the nasal provocation test (NPT) (11, 12). Moreover, these compounds induced or augmented eosinophilic inflammation in both early and late responses.
In this study, we performed immunoassays to measure the levels of AAT in the NLFs following challenge with Dermatophagoides pteronyssinus (Dpt). The goal of this study was to determine the involvement of AAT in the nasal allergic inflammation. In addition, the association of AAT with eosinophil activation and locally produced Dpt-specific IgA antibodies was investigated. Furthermore, immunohistochemical studies were done using AAT antibodies to assess the localization and differential distribution of AAT in the nasal mucosa.
Forty subjects were mono-sensitized to Dpt by a skin prick test using 50 inhalant allergens (Bencard, Bradford, UK). Among them, 20 patients were diagnosed with symptomatic AR, based on their clinical history, skin prick test, and a positive response on the NPT with the Dpt allergen. These individuals were frequently bothered in daily life with rhinitis symptoms including rhinorrhea, itching sensation, nasal obstruction, and sneezing, and were designated Group I. The other 20 asymptomatic subjects with a negative NPT response were considered asymptomatic AR patients (Group II). None of the subjects had a history of upper airway infection or medication, including antihistamines, steroids, leukotriene receptor antagonists, or nasal spray for 4 weeks before the study. The subjects with asthma, chronic rhinosinusitis, septal deviation, nasal polyps, or a history of immunotherapy were excluded from this study. All subjects were non-smokers. This study was approved by the Institutional Review of Board of Ajou Medical Center, Suwon, Korea, and informed consent was obtained from all subjects.
NPT was performed in all subjects using the Dpt allergen as previously described (11). Briefly, all subjects visited the clinic in the morning and were seated in a room maintained at room temperature for 30 minutes to minimize the effects of the stimuli from daily-life. Before the NPT, a saline challenge was performed to exclude nasal hyperreactivity. An 8-mm filter paper disc (punched from a Shandon filter card; Pittsburgh, PA, USA) soaked with the allergen solution (5,000 BU/Ml Dpt, prepared by Allergopharma, Reinbek, Germany) was placed onto the anterior tip of the inferior turbinate on the wider side of the nose for 10 minutes. Nasal symptom scores were determined at baseline, and at 10 minutes, 30 minutes, 3 hours, and 6 hours postprovocation. Rhinorrhea, nasal itching, and nasal obstruction symptoms were rated on an arbitrary scale from 0 to 3 (0, no symptoms; 1, mild symptoms; 2, moderate symptoms; 3, severe symptoms). Sneezes were counted and classified as follows: 0, no sneezing; 1, 1 to 4 sneezes; 2, 5 to 9 sneezes; 3, ≥ 10 sneezes. A positive response was defined as an increase of more than three points for the total nasal symptom scores from the sum of the rhinorrhea, itching, nasal obstruction, and sneezing symptom scores during the provocation test.
NLF sampling was performed bilaterally before the allergen provocation and at 10 minutes, 30 minutes, 3 hours, and 6 hours after the NPT as previously described (12). The subject's head was tilted backward to 30° to close the nasopharynx with the soft palate. Five milliliters of saline was instilled into one nostril with a syringe. After 10 seconds, the subject was asked to flex the neck and expel the solution into a polypropylene tube. This process was again performed for the contralateral nostril. The nasal secretions were immediately placed on ice to increase cell viability. The samples were centrifuged at 300 g for 10 minutes. The cell-free supernatants were stored at -80℃ until further processing, while the cell pellets were used to prepare slides, which were stained using the May-Grunwald and Giemsa procedures to morphologically assess the cells in the fluid. The percentages of eosinophils and other inflammatory cells were calculated as the number of cells per 100 cells on a slide.
The level of AAT (Immunodiagnostic Systems, Fountain Hills, AZ, USA) in each NLF sample and serum was measured using an enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's instructions. The level of eosinophil cationic protein (ECP) in each NLF sample was measured using the ImmunoCAP system (Pharmacia-Upjohn, Uppsala, Sweden). The minimal detection limit for the ECP was 2 ng/mL. The levels of Dpt-specific IgA antibodies in each NLF sample were determined by ELISA as previously described (11, 12).
Nasal mucosa biopsy specimens of the inferior turbinate were obtained from four patients in Group I and five in Group II without nasal provocation. In brief, 4 µm-thick sections of formalin-fixed and paraffin-embedded tissues were deparaffinized with Bond Dewax Solution (Vision BioSystems, Mount Waverley, Victoria, Australia), and an antigen retrieval procedure was performed using Bond ER Solution (Vision BioSystems) for 20 minutes at 100℃. Endogenous peroxidase was quenched by incubation with hydrogen peroxide for 15 minutes. Sections were incubated in a Bond-max automatic slide stainer (Vision BioSystems) for 15 minutes at ambient temperature with a 1:2,000 dilution of primary antibody against human AAT (rabbit polyclonal anti-human AAT, Zymed Laboratories, San Francisco, CA, USA). Next, we applied a biotin-free polymeric horseradish peroxidase (HRP)-linker antibody conjugate system for 10 minutes followed by chromogen (3,3'-diaminobenzidine) for 5 minutes and a hematoxylin and eosin (H&E) counterstain. Appropriate positive and negative controls were included in each immunohistochemistry run. All immunohistochemically stained slides were interpreted by two independent investigators (JHL and YWK) without knowledge of the clinicopathologic information. The intensity of the cytoplasmic staining of AAT was graded according to the following scale: 0, no staining; 1+, mild staining; 2+, moderate staining, and 3+, marked staining.
A Mann-Whitney U-test was used for comparison between the groups, and a Wilcoxon signed rank test was also used to compare the results obtained according to the time interval after the NPT within the group. Spearman correlations were applied to define the relationships between the AAT and ECP, and the Dpt-specific antibodies. All analyses were performed using SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). P-values ≤0.05 were regarded as statistically significant.
The clinical characteristics of the two groups are shown in Table 1. There were no significant differences in gender, age, skin reactivity to Dpt, and the level of Dpt-specific IgE in the serum between the two groups (all P>0.05). In addition, no significant difference was noted in the level of AAT in serum between the two groups (P>0.05).
At baseline, the level of Dpt-specific IgA in the NLFs of Group I were significantly higher than those of Group II (P=0.009), while no significant difference was noted in the levels of ECP and AAT between the two groups (P=0.051 and P=0.186, respectively). After the NPT, all ECP levels in the NLFs of Group I at 10 minutes, 30 minutes, 3 hours, and 6 hours were significantly higher than those of Group II (P=0.013, P=0.049, P=0.008, and P=0.020, respectively). In addition, the levels of AAT and Dpt-specific IgA in the NLFs of Group I at all time intervals, except at 30 minutes, were significantly higher than those of Group II (AAT; P=0.003, P=0.066, P=0.035, and P=0.009, respectively: Dpt-specific IgA; P<0.001, P=0.074, P=0.022, and P=0.005, respectively) (Fig. 1). The percentage of eosinophils was significantly higher in the NLFs of Group I at all times after the NPT compared to Group II (P=0.047, P=0.019, P=0.013, and P=0.032, respectively), while no significant difference was noted at baseline between the two groups (P=0.472). In contrast, the percentage of neutrophils in the inflammatory cell counts was significantly higher in the NLFs of Group II at baseline, 3 hours, and 6 hours after the NPT compared to Group I (P=0.036, P=0.005, and P=0.047, respectively).
There was a significant correlation between the levels of AAT and ECP in the NLFs at baseline and all time intervals after the NPT (r=0.447, P=0.006; r=0.423, P=0.013; r=0.498, P=0.002; r=0.530, P=0.001; and r=0.600, P<0.001, respectively). Furthermore, the level of AAT significantly correlated with the level of Dpt-specific IgA in the NLFs at all times after the NPT (r=0.374, P=0.022; r=0.480, P=0.003; r=0.593, P<0.001; r=0.722, P<0.001; and r=0.587, P<0.001, respectively) (Fig. 2).
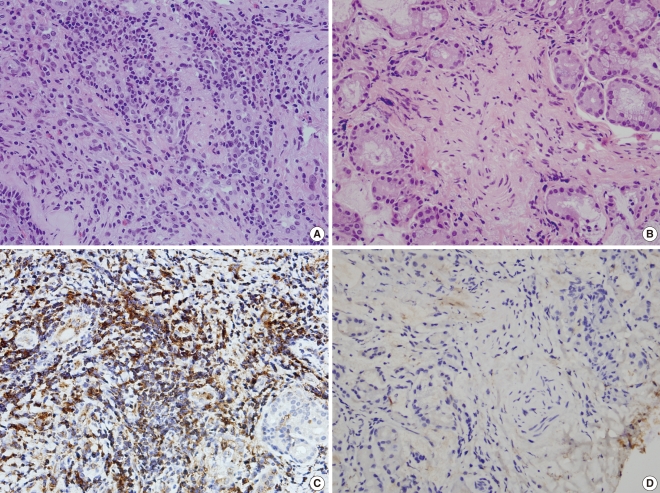
Expression of AAT in nasal mucosa by immunohistochemical staining is shown in Fig. 3. Cytoplasmic expression of AAT protein was noted in nasal epithelium and stromal cells, as well as in the inflammatory cells. In Group I, all cases were positive for AAT. In contrast, the nasal mucosa from Group II showed very weak staining. When we scored expression of AAT from 0-3 according to the staining intensity, the mean score of Group I was significantly higher than that of Group II (2.25±0.96 vs. 0.4±0.55, P=0.032).
We evaluated the changes in the AAT levels in the NLFs at specific time intervals after a Dpt allergen challenge in two groups and studied the symptoms in response to the NPT. The AAT levels measured at all time intervals after the NPT, except at 30 minutes, were significantly higher in the symptomatic AR group compared to the asymptomatic AR group, although no significant differences were observed before the NPT between the two groups (Fig. 1). The AAT level at 10 minutes was the highest, followed by those at 30 minutes, 3 hours, 6 hours, and baseline; the levels at 10 and 30 minuntes were significantly higher compared to the baseline levels.
A few studies have reported that AAT levels were increased in the NLFs of patients with asthma or AR after allergen or pine particle challenges (9, 10, 13). A prior proteomic analysis in the NLFs of patients with seasonal AR, before and during allergy season, compared the results with healthy controls (10); the AAT levels in the proteomic analysis were higher in the patients with AR during both seasons compared to the controls. The increase in the AAT levels can be explained by its role in protecting the airway from the proteolytic damage caused by neutrophil elastase.
For the first time, we demonstrate the involvement of AAT in allergen-induced nasal inflammation. AR is characterized by an IgE-dependent release of mediators from infiltrating inflammatory cells, such as mast cells and eosinophils. Among the inflammatory cells involved in allergic inflammation, eosinophils are the most important effector cells in the nasal secretion of patients with AR (14, 15). In addition, allergen-specific antibodies might be a contributing factor in allergic airway inflammation (16). Previously, we found that Dpt-specific IgA and ECP levels in the NLFs following Dpt allergen challenge were increased in patients with AR, with the concentration of Dpt-specific IgA and ECP levels displaying a close relationship. These findings suggested that locally produced allergen-specific IgA may be involved in the eosinophil activation found in allergen-induced nasal inflammation (11, 12). In the present study, the increase in the AAT levels was accompanied by an increase in the Dpt-specific IgA and ECP levels during the NPT. Consistent with the findings of previous studies, the Dpt-specific IgA level significantly correlated with the ECP level at all time intervals studied with the NPT. Furthermore, the AAT level significantly correlated with ECP level at all time points and with Dpt-specific IgA at the early time intervals studied with the NPT (Fig. 2). The AAT and Dpt-specific IgA levels peaked at 10 minutes after the Dpt allergen challenge, in contrast to the ECP level that peaked at 30 minutes. These results suggest that the earlier increase of AAT and Dpt-specific IgA levels may lead to activation or augmentation of eosinophils in nasal allergic inflammation.
The major source of AAT in the body is from the hepatocytes and, to a lesser extent, it is synthesized by monocyte/macrophages, activated lymphocytes, and epithelial cells (4-6). AAT, as an acute phase protein, may increase 3-4 fold during an acute inflammatory reaction, most of which is found in the plasma (17). Many investigators have tried to explain how AAT could be synthesized and released in local inflammation reactions at sites not directly in contact with the plasma. According to recent studies, activated neutrophils and eosinophils can store and secrete AAT, which may play a role in main protection of tissues from neutrophil elastase at local inflammation sites (7, 8). Johansson et al. (8) demonstrated the presence of AAT in the specific granules of eosinophils by immunoelectron microscopy; in addition, the AAT contents in the lysates of highly purified eosinophils were found to be much higher than in neutrophils and monocytes. In this study, we demonstrate the presence of AAT in the inflammatory cells of the nasal mucosa from symptomatic subjects with AR by H&E and immunohistochemical staining (Fig. 3). The H&E stained slides showed that the nasal mucosa from symptomatic subjects with AR had a much higher proportion of infiltrating inflammatory cells, including monocytes, lymphocytes, and plasma cells, as well eosinophils, as compared to the controls. Cells immunostained for AAT were brown colored and were similar to the inflammatory cells observed by H&E staining, but not at submucosal glands, substrate cells, or submucosal capillaries. In addition, the epithelial cells in the nasal mucosa were stained weakly. These results suggest that the secretion of AAT in allergen-induced nasal inflammation may originate mainly from the inflammatory cells and, to a lesser extent, from the epithelial cells. The immunoassay and immunostaining studies revealed that more eosinophils and other inflammatory cells, as the main source of AAT, were infiltrated and then activated in the nasal mucosa of symptomatic AR patients compared with asymptomatic AR patients. These observations would explain the difference of AAT level between symptomatic and asymptomatic AR patients.
Although we detected a local burst of AAT secretion in response to the allergen challenge, we did not elucidate the role and effect of the AAT after it was secreted. This is a limitation of this study. It is well known that AAT plays a role in inhibiting neutrophil elastase, and thus contributes to the protection of the airway from proteolytic damage. The inactivation of AAT and the distortion of the proteinase/antiproteinase balance in allergic airway inflammation are supported by the following reports. First, eosinophils recruited into the tissue following an allergen challenge produce reactive oxygen species, which results in numerous proteins being oxidized and inactivated (18). AAT is the main oxidized protein in the bronchoalveolar lavage fluid after an allergen challenge in patients with atopic asthma (19, 20). Der Dpt p1, a cystein proteinase derived from the species Dpt, cleaves and inactivates the reactive loop of AAT (21). Moreover, cleavage of the AAT reactive loop might be chemotactic for human neutrophils and, consequently, it might exacerbate tissue damage and accentuate allergic inflammation (22). Der Dpt p1 is a major component of the allergic immune response to house dust mites in atopic individuals, by virtue of the increased permeability of allergens in the respiratory tract (23) and cleavage of the low affinity receptor for IgE, which results in the disruption of the regulation of IgE synthesis (24).
In spite of the limitations of this study, the findings suggest that the Dpt allergen penetrates the epithelial layer during the early phase of the NPT and induces the secretion of AAT from epithelial and inflammatory cells. Immediately thereafter, the Dpt allergen and reactive oxygen species secreted from the activated eosinophils inactivated AAT by cleavage and oxidation.
In this study, we evaluated the changes of AAT in the NLFs following an allergen challenge. The results show that AAT levels are much higher in the NLFs from symptomatic patients with AR than in asymptomatic patients. Moreover, AAT secretion with the NPT, especially during the early phase, is closely related with allergic inflammatory mediators, such as ECP and Dpt-specific IgA antibodies. In addition, immunohistochemical staining revealed the storage of AAT in the infiltrating inflammatory cells of the nasal mucosa and secretion in the local response to allergen stimulation.
Patients with symptomatic allergic rhinitis secrete a burst of AAT in response to allergenic stimulation; this response was observed to be closely associated with the activation of eosinophils induced by allergen-specific IgA. In allergen-induced nasal inflammation, AAT might be a byproduct of the activated inflammatory cells, and is thus implicated in the allergic immune response.
ACKNOWLEDGMENT
This study was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MEST) (2009-0078646).
References
1. Naclerio RM, Proud D, Peters SP, Silber G, Kagey-Sobotka A, Adkinson NF Jr, et al. Inflammatory mediators in nasal secretions during induced rhinitis. Clin Allergy. 1986; 3. 16(2):101–110. PMID: 3708789.

2. Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997; 5. 15. 89(10):3503–3521. PMID: 9160655.

3. Travis J, Salvesen GS. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983; 52:655–709. PMID: 6193754.

4. Hubbard R, Crystal RG. Crystal RG, West JB, editors. Antiproteases. The lung. 1991. New York: Raven Press;p. 1775–1788.
5. Cichy J, Potempa J, Travis J. Biosynthesis of alpha1-proteinase inhibitor by human lung-derived epithelial cells. J Biol Chem. 1997; 3. 28. 272(13):8250–8255. PMID: 9079644.
6. Bergman D, Kadner SS, Cruz MR, Esterman AL, Tahery MM, Young BK, et al. Synthesis of alpha 1-antichymotrypsin and alpha 1-antitrypsin by human trophoblast. Pediatr Res. 1993; 9. 34(3):312–317. PMID: 8134173.
7. Paakko P, Kirby M, du Bois RM, Gillissen A, Ferrans VJ, Crystal RG. Activated neutrophils secrete stored alpha 1-antitrypsin. Am J Respir Crit Care Med. 1996; 12. 154(6 Pt 1):1829–1833. PMID: 8970377.

8. Johansson B, Malm J, Persson T, Janciauskiene S, Andersson P, Carlson J, et al. Alpha-1-antitrypsin is present in the specific granules of human eosinophilic granulocytes. Clin Exp Allergy. 2001; 3. 31(3):379–386. PMID: 11260148.

9. Westin U, Lundberg E, Wihl JA, Ohlsson K. The effect of immediate-hypersensitivity reactions on the level of SLPI, granulocyte elastase, alpha1-antitrypsin, and albumin in nasal secretions, by the method of unilateral antigen challenge. Allergy. 1999; 8. 54(8):857–864. PMID: 10485390.
10. Ghafouri B, Irander K, Lindbom J, Tagesson C, Lindahl M. Comparative proteomics of nasal fluid in seasonal allergic rhinitis. J Proteome Res. 2006; 2. 5(2):330–338. PMID: 16457599.

11. Oh JH, Hur GY, Ye YM, Kim JE, Park K, Park HS. Correlation between specific IgA and eosinophil numbers in the lavage fluid of patients with perennial allergic rhinitis. Allergy Asthma Proc. 2008; Mar–Apr. 29(2):152–160. PMID: 18430312.

12. Choi GS, Park HJ, Hur GY, Choi SJ, Shin SY, Ye YM, et al. Vascular endothelial growth factor in allergen-induced nasal inflammation. Clin Exp Allergy. 2009; 5. 39(5):655–661. PMID: 19236408.

13. Nikasinovic L, Just J, Sahraoui F, Seta N, Grimfeld A, Momas I. Nasal inflammation and personal exposure to fine particles PM2.5 in asthmatic children. J Allergy Clin Immunol. 2006; 6. 117(6):1382–1388. PMID: 16751001.

14. Nouri-Aria KT, O'Brien F, Noble W, Jabcobson MR, Rajakulasingam K, Durham SR. Cytokine expression during allergen-induced late nasal responses: IL-4 and IL-5 mRNA is expressed early (at 6 h) predominantly by eosinophils. Clin Exp Allergy. 2000; 12. 30(12):1709–1716. PMID: 11122208.
15. Gelfand EW. Inflammatory mediators in allergic rhinitis. J Allergy Clin Immunol. 2004; 11. 114(5 Suppl):S135–S138. PMID: 15536444.

16. Benson M, Reinholdt J, Cardell LO. Allergen-reactive antibodies are found in nasal fluids from patients with birch pollen-induced intermittent allergic rhinitis, but not in healthy controls. Allergy. 2003; 5. 58(5):386–392. PMID: 12752324.

17. Carlson J, Eriksson S. Alpha 1-antitrypsin and other acute phase reactants in liver disease. Acta Med Scand. 1980; 207(1-2):79–83. PMID: 6966119.
18. Owen S, Pearson D, Suarez-Mendez V, O'Driscoll R, Woodcock A. Evidence of free-radical activity in asthma. N Engl J Med. 1991; 8. 22. 325(8):586–587. PMID: 1857396.

19. Foreman RC, Mercer PF, Kroegel C, Warner JA. Role of the eosinophil in protein oxidation in asthma: possible effects on proteinase/antiproteinase balance. Int Arch Allergy Immunol. 1999; Feb–Apr. 118(2-4):183–186. PMID: 10224372.

20. Nagai K, Betsuyaku T, Konno S, Ito Y, Nasuhara Y, Hizawa N, et al. Diversity of protein carbonylation in allergic airway inflammation. Free Radic Res. 2008; 11. 42(11-12):921–929. PMID: 19031315.

21. Kalsheker NA, Deam S, Chambers L, Sreedharan S, Brocklehurst K, Lomas DA. The house dust mite allergen Der p1 catalytically inactivates alpha 1-antitrypsin by specific reactive centre loop cleavage: a mechanism that promotes airway inflammation and asthma. Biochem Biophys Res Commun. 1996; 4. 05. 221(1):59–61. PMID: 8660343.
22. Banda MJ, Rice AG, Griffin GL, Senior RM. The inhibitory complex of human alpha 1-proteinase inhibitor and human leukocyte elastase is a neutrophil chemoattractant. J Exp Med. 1988; 5. 01. 167(5):1608–1615. PMID: 3259253.

23. Herbert CA, Holgate ST, Robinson C, Thompson PJ, Stewart GA. Effect of mite allergen on permeability of bronchial mucosa. Lancet. 1990; 11. 03. 336(8723):1132. PMID: 1978008.

24. Hewitt CR, Brown AP, Hart BJ, Pritchard DI. A major house dust mite allergen disrupts the immunoglobulin E network by selectively cleaving CD23: innate protection by antiproteases. J Exp Med. 1995; 11. 01. 182(5):1537–1544. PMID: 7595223.

Fig. 1
Changes in alpha1-antitrypsin (A), ECP (B), and Dpt-specific IgA antibody (C) in nasal lavage fluid during the nasal provocation test with Dpt allergen. Solid circles, Group I; open circles, Group II. The results are expressed as the mean±SD. *P<0.05 (Group I vs. Group II), †P<0.05 (baseline vs. level at elapsed time after challenge).
ECP: eosinophilic cationic protein; HDM: house dust mite; Dpt: Dermatophagoides pteronyssinus.
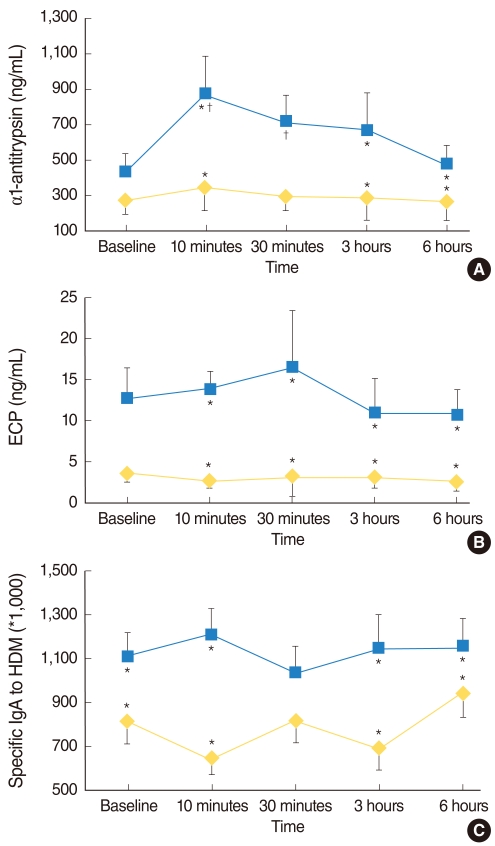
Fig. 2
Correlation between the alpha1-antitrypsin (AAT) levels and eosinophilic cationic protein (ECP) levels collected before (A), 30 minutes after (B), and 6 hours (C) after the nasal provocation test, or Dermatophagoides pteronyssinus-specific IgA levels collected before (D), 3 minutes (E) and 6 hours (F) after the nasal provocation test in the allergic rhinitis group. r: Spearman's correlation coefficient; HDM: house dust mite.
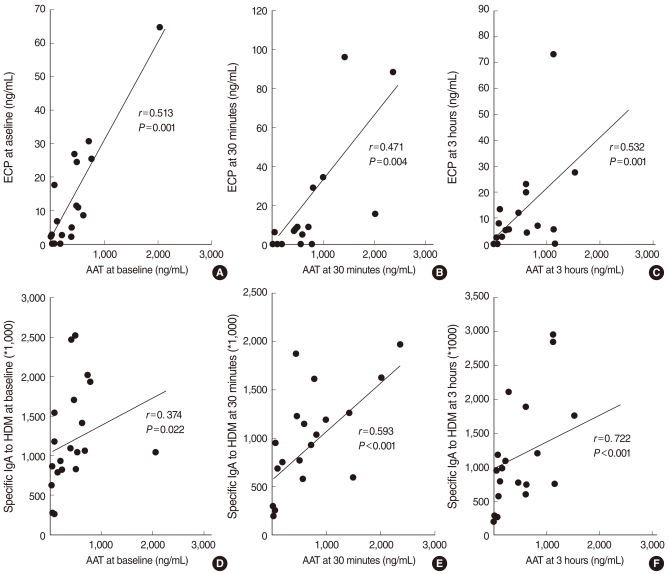
Fig. 3
The nasal mucosa of Group I (A) was infiltrated by inflammatory cells in the stroma, In contrast, the nasal mucosa of Group II (B) showed a few inflammatory cells scattered. (H&E, ×200). Immunohistochemical expression of alpha1-antitrypsin (AAT) in nasal mucosa. Diffuse and strong expression of AAT in Group I (C). Negative expression of AAT in Group II (D) (×200).




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download