Abstract
Objectives
Acinic cell carcinoma (AciCC) is a rarely encountered malignancy in parotid gland. Because AciCC is rare and was recently recognized as the entity of malignancy, AciCC has been difficult to study. We aimed to analyze the diagnosis and treatment experience for this malignancy in our hospital.
Methods
We retrospectively reviewed medical records of the 20 patients with AciCC of parotid gland diagnosed from 1990 to 2009. The preoperative computed tomography scan, preoperative fine needle aspiration cytology (FNAC) and intraoperative frozen section results were compared with the final diagnosis. The survival and recurrence were analyzed with the cancer stages and treatment modalities.
Results
There were 10 males and 10 females, with a mean age of 44.4 years, ranging 8-77 years. The AJCC tumor stage distributions of the patients were 70%, 15%, and 15% for stages I, II, and IV, respectively. The sensitivity of FNAC and intraoperative frozen section was 26.7% and 50.0% respectively. The 10-year survival rate was 90.9% with a mean follow-up of 111 months, ranging 17-251 months. The 10-year disease free survival rate was 74.2% and the mean duration of recurrence from initial surgery was 92.3 months.
Conclusion
AciCC of the parotid gland is a rare malignancy that has features of less aggressive behavior, and good prognosis. Intraoperative frozen section examination may be helpful in the diagnosis of AciCC of the parotid gland because of the low sensitivity of preoperative computed tomography scan and FNAC. Surgery with adjuvant postoperative radiotherapy is satisfactory for disease control.
Acinic cell carcinoma (AciCC) is a relatively uncommon malignancy accounting for 1 to 6% of all salivary gland tumors and 15% of all malignant tumors in parotid gland (1, 2). Interestingly, AciCC was originally described as an adenoma, but from the 1950s the term acinic cell adenocarcinoma was coined when its ability to metastasize and recur locally had been recognized (3, 4). It is hard to diagnose AciCC of parotid gland preoperatively. Computed tomography (CT) scan demonstrated a low sensitivity rate for malignancy. The yield of fine needle aspiration cytology (FNAC) was not high enough to make a correct prediction for AciCC (5). The results of AciCC treatment are quite variable. Local recurrence rate has been reported in 8 to 56% of the patients (5). Mortality from the disease is usually less than 10% at 5 years (6).
Because AciCC is rare and was recently recognized as the entity of malignancy, AciCC has been difficult to study. In this study, we retrospectively reviewed 20 parotid AciCC patients undergoing surgery from 1990 to 2009. We aimed to analyze the diagnosis and treatment experience for this malignancy in our hospital.
We retrospectively reviewed the charts of 20 patients diagnosed as parotid AciCC in the Department of Otorhinolaryngology at the Seoul National University Hospital from 1990 to 2009. It was conducted with the approval of institutional review board of SNUH. All patients received surgical intervention with a diagnosis of tumor of the parotid gland, which was confirmed pathologically. The records were analyzed for age, sex, clinical features, duration of symptoms, facial palsy, FNAC, imaging study (CT scan), intraoperative frozen section examination, treatment modalities, histopathologic findings, survival, recurrence. The staging system was based on the 7th American Joint Committee on Cancer staging criteria (7). Postoperative radiotherapy (RT) was performed on patients with advanced tumor stage or lack of confidence as to safety surgical margins. Chemotherapy was performed on one patient with distant metastasis.
SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Overall cumulative survival rate and disease free survival rate was calculated with Kaplan-Meier method.
This study comprised 20 patients diagnosed as parotid AciCC. There were 10 males and 10 females, with a mean age of 44.4 years (range, 8 to 77 years). No significant gender predominance was found in this study. Mean follow-up duration was 111 months (range, 17 to 251 months). Tumors were mainly located in the superficial lobe (12 cases, 60%), while there were 8 (40%) in deep lobe. Among all patients, infra-auricular mass was the most common initial presentation (95%). There was no patient that complained facial palsy preoperatively.
CT scans were performed in all patients. The radiologists in charge of interpretation of CT scan reported following four statements - benign mass lesion (8 cases, 40%), more possible benign lesion (5 cases, 25%), more possible malignancy (1 cases, 5%), and malignancy (6 cases, 30%).
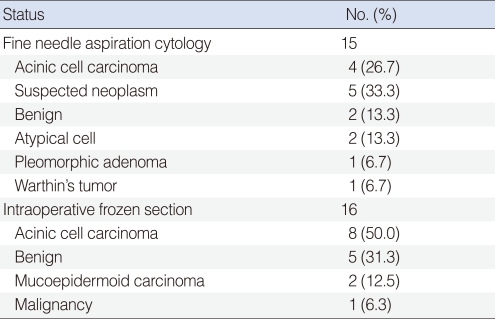
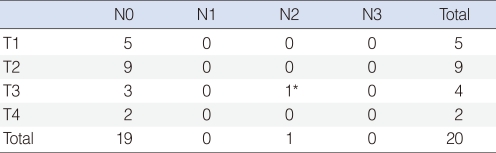
Fifteen patients had preoperative FNAC. Four cases (26.7%) had a cytology diagnosis that was consistent with AciCC. Instead of a correct diagnosis, suspected neoplasm (5 cases, 33.3%), benign (2 cases, 13.3%), atypical cell (2 cases, 13.3%), pleomorphic adenoma (1 case, 6.7%) and Warthin's tumor (1 case, 6.7%) were reported. Intraoperative frozen section were examined in 16 patients, which offered 8 (50%) diagnoses that were consistent with AciCC (Table 1).
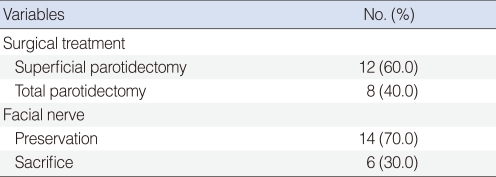
All patients underwent surgical intervention of parotid gland tumor. Twelve patients underwent superficial parotidectomy and 8 had total parotidectomy. Facial nerve dissection and preservation were accomplished in 14 patients. In 6 patients, facial nerve was sacrificed without reconstruction due to advanced stage or tumor involvement. One patient underwent sural nerve graft reconstruction after facial nerve resection at the same operation. One patient with level II lymph node involvement and lung metastasis underwent superficial parotidectomy, ipsilateral radical neck dissection and lung lobectomy (Table 2).
The T staging was summarized as follows: T1 in 5 (20%) patients, T2 in 9 (45%) patients, T3 in 4 (20%) patients, and T4 in 2 (10%) patients. One patient had cervical lymph node involvement and lung metastasis (Table 3). The AJCC tumor stage distributions of the patients were 14 (70%), 3 (15%), and 3 (15%) for stages I, II, and IV, respectively.
After surgery of parotid AciCC, postoperative radiotherapy was given in 8 patients, and adjuvant concurrent chemoradiotherapy was used in 1 patient.
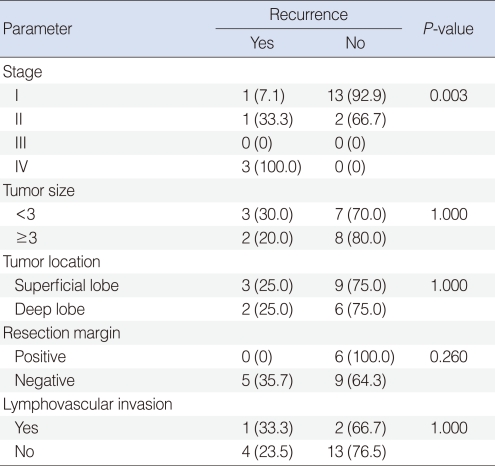
There are 5 cases of recurrence after surgery with or without adjuvant treatment. Three cases of distant metastasis occured. Among them, 2 patients developed lung and one brain metastasis were in 2 and 1 respectively. Two patients had local recurrence in tumor bed. Overall stage was correlated with recurrence. However, there is no significant relationship with tumor size, tumor location, resection margin and lymphovascular invasion (Table 4). One patient initially was diagnosed with lung metastasis died of brain metastasis after the operation and concurrent chemoradiotherapy.
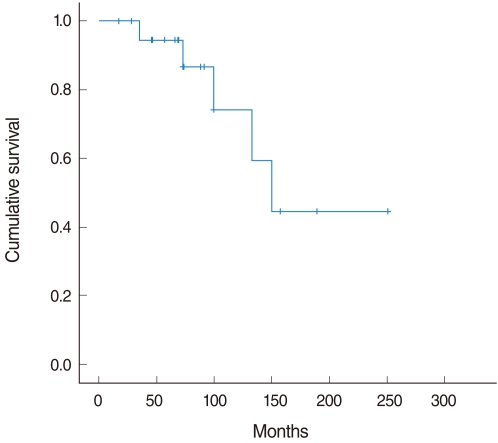
The 10-year cumulative survival rate was 90.9% with a mean follow-up of 111 months, ranging 17-251 months. The 10-year disease free survival rate was 74.2% and the mean duration of recurrence from initial surgery was 92.3 months (Fig. 1).
AciCC is a rare malignancy. It was initially thought to be benign disease entity and Foote and Frazell (3) first coined it as a carcinoma in the early 1950s. Its ability to metastasize and recur locally was first reported. Because of its rarity, initial misunderstanding and unknown character of AciCC, it has been difficult to study (8).
Patients usually visit doctors with the symptom of incidental infra-auricular mass. Facial palsy or pain usually may not exist at the first time. In other studies, the tumor usually appeared as a painless mass in either the superficial or deep lobe of the parotid gland (9-13). In our study, most of the patients visited for infra-auricular mass and there was no patient that complained facial palsy preoperatively. It was thought that the slow growth pattern of AciCC made the mass painless.
In the literature, the female-to-male ratio is about 1.5:1, and the tumor usually occurs in the fifth and sixth decades of life (14). Most of studies have shown females predominant to males at a higher rate. Exceptionally Ellis and Corio (12) suggested that 155 of 294 patients were male (53%). In our study, there was no predominance of gender. A mean age of 44.4 years at diagnosis in our study was younger than that found in past ones.
Preoperative imaging studies are widely used for head and neck tumors. In our study, CT scan demonstrated a low sensitivity rate for malignancy like FNAC. Six cases were suspected with malignancy preoperatively by CT scan. There was no unique characteristic of parotid gland AciCC in the CT scan. Fine needle aspiration cytology could help clinicians in the differential diagnosis of parotid malignancy from other benign tumors. As for AciCC of parotid gland, the diagnostic ability of FNAC is not good. In other study, 12 patients had preoperative fine-needle aspiration, while only two cases (17%) were consistent with correct cytology diagnosis for AciCC (5). From our experience, the yield of FNAC (4 of 15 cases, 26.7%) was not high enough to make a correct diagnosis of AciCC preoperatively. An adjuvant diagnostic tool for diagnosis of parotid AciCC was necessary, so we performed intraoperative frozen section biopsy and examination. In our result, the sensitivity of intraoperative frozen section itself was 50% and when performed simultaneously with FNAC, its sensitivity was 69.2%.
The surgical treatment of choice is total or superficial parotidectomy with dissection of the facial nerve according to mass location. Facial nerve preservation was intra-operatively attempted in all patients except those with macroscopic nerve involvement. If the tumor was well encapsulated without extra-capsular spread, Gomez et al. (15) suggested that surgery alone was satisfactory in the control of AciCC. Positive surgical margin was regarded as an important prognostic factor in AciCC (15). However, from our analysis, there was no recurrence in all cases with positive resection margin.
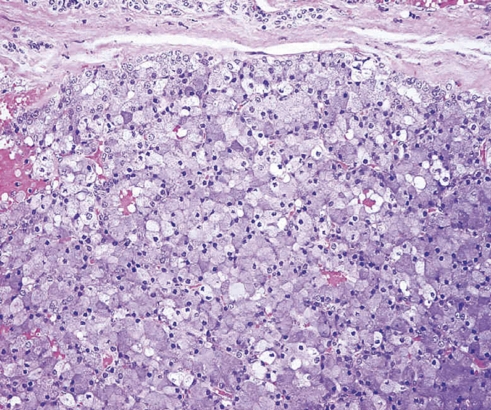
AciCC is considered a low-grade salivary gland carcinoma with low rate of recurrence and metastasis, and is generally thought to carry a good prognosis. It usually presents as a single or multilobular mass. Cystic degeneration or hemorrhage may be seen in histopathology. The neoplastic cells have morphologic similarity with the normal acinic cells. The pathological diagnosis of AciCC depends on identifying cells presenting serous acinar cell differentiation (Fig. 2).
Immunohistochemical staining of AciCC is not specific and does not play an important role in diagnosis. Neural, vessel, or stromal invasion is rare and regarded as a poor prognostic factor (15). Gomez et al. (15) reported that high-grade AciCC with the features of tumor necrosis and high mitotic figures were correlated with poor disease free survival. But the information of nerve involvement, stromal invasion, high mitotic figures and tumor necrosis were not mentioned in our pathologic report.
The cervical lymph node metastatic rate in AciCC was reported to be 2.86% with N1 and 5.71% with N2b lesions and the rate of occult regional metastasis was low (15). In our study, regional metastasis in level IIb was found in one patient (1 of 20 patients, 5%). To date, the role of neck dissection in AciCC remains controversial. Therapeutic neck dissection is the accepted treatment for patients with clinically obvious cervical nodal involvement. However, the elective neck dissection of the clinically negative neck is not well defined in the published work. The role of elective neck dissection was not evident in our study or in other studies (15). Some authors suggest routine elective neck dissection in all N0 parotid carcinomas regardless of histology (16). Stennart et al. (17) also recommended a similar treatment, because most salivary gland malignancy including AciCC are not radiosensitive. However, no survival gain has been shown in these two studies. In contrast, several other authors do not believe that routine neck dissection is recommended (6, 12, 18, 19). In our study, we did not perform elective neck dissection on the clinically negative neck and there was no regional treatment failure. In one case of cervical lymph node involvement with lung metastasis, we performed the ipsilateral radical neck dissection along with superficial parotidectomy and lung lobectomy for oncologic safety.
There has been no prospective randomized study to evaluate the effectiveness of radiotherapy. Primary radiotherapy should be restricted to patients not suitable for surgery or refusing surgery because AciCC has generally not been regarded as being radiosensitive. Some authors have only found a limited response (18, 20). Others have claimed a response to postoperative radiotherapy (9, 21). Spafford et al. (9) proposed a series of indications for postoperative adjunctive radiotherapy in AciCC: 1) recurrent tumor; 2) equivocal or positive margins, or evidence of tumor spillage; 3) tumor adjacent to the facial nerve; 4) deep-lobe involvement; 5) lymph node metastases; 6) extra-parotid extension; and 7) large tumors greater than 4 cm. Greig et al. (22) suggested that the indications for postoperative radiotherapy used were the presence of high-grade histological features (evidence of lympho-vascular invasion and high mitotic rate), close or positive margins at initial resection and tumor recurrence.
In our study, patients with advanced stage or positive resection margin were given adjuvant postoperative radiotherapy and had good tumor control. We recommend giving adjuvant radiation therapy in these high-risk patients. There are few studies about the effectiveness of chemotherapy in parotid AciCC. In our study, we adopted chemotherapy as adjuvant therapy in one patient with lung metastasis but after several recurrences, the patient died of brain metastasis. We could not evaluate the outcome of chemotherapy due to limited cases in this study.
It was reported that local recurrence rates ranged from 0 to 44%. Reported cervical nodal metastases have ranged from 0% to 43%. Distant metastases have ranged from 0 to 13% (1, 9, 12, 18-20, 23). In our study, local recurrence rate and distant metastases were 10% and 15%. There was no regional recurrence after treatment. The cases of distant metastasis had more aggressive clinical feature than others. They were suspicious for high grade AciCC but we could not found the pathologic reports about tumor necrosis and high mitotic figures.
AciCC had better prognosis relative to other malignancies, with 10- and 20-year survivals of 88% and 83%, respectively in the literature (24). The prognosis of AciCC is variable from many series studies, ranging from 55 to 89% (15, 20, 21). Eneroth et al. (21) reported that the cure rate decreased from 89% at 5 years to 56% at 20 years. Spiro et al. (20) reported the survival rate to be 76% at 5 years, 63% at 10 years, and 55% at 15 years. According to our data, prognosis was good, and the 10-year disease free survival rate was 74.2% and the overall 10-year survival rate was 90.9%.
AciCC has been noted to recur many years after initial diagnosis and surgery. Spiro (25) reported two cases recurring beyond 30 years of follow-up, a median time to recurrence of 3 years, and median time to death from disease of 10 years. However, the large study of 294 patients by Ellis and Corio (12) showed that 82% of first recurrences and metastases occurred within 5 years of initial therapy. Two patients recurred after 11 and 13 years respectively in our patients. Other authors have maintained that a follow-up of at least 20 years is necessary to adequately determine final treatment outcomes (9, 26).
In conclusions, parotid AciCC is a rare malignancy that has features of less aggressive behavior, and good prognosis. Intraoperative frozen section examination may be helpful in the diagnosis of parotid AciCC because of the low sensitivity of preoperative CT scan and FNAC. Surgery with adjuvant postoperative radiotherapy is satisfactory for disease control.
References
1. Laskawi R, Rodel R, Zirk A, Arglebe C. Retrospective analysis of 35 patients with acinic cell carcinoma of the parotid gland. J Oral Maxillofac Surg. 1998; 4. 56(4):440–443. PMID: 9541342.

2. Batsakis J. Tumors of the head and neck: clinical and pathological considerations. 1979. 2nd ed. Baltimore: Williams & Wilkins.
3. Foote FW Jr, Frazell EL. Tumors of the major salivary glands. Cancer. 1953; 11. 6(6):1065–1133. PMID: 13106826.

4. Buxton RW, Maxwell JH, French AJ. Surgical treatment of epithelial tumors of the parotid gland. Surg Gynecol Obstet. 1953; 10. 97(4):401–416. PMID: 13102167.
5. Lin WN, Huang HC, Wu CC, Liao CT, Chen IH, Kan CJ, et al. Analysis of acinic cell carcinoma of the parotid gland: 15 years experience. Acta Otolaryngol. 2010; 12. 130(12):1406–1410. PMID: 20809887.
6. Kim SA, Mathog RH. Acinic cell carcinoma of the parotid gland: a 15-year review limited to a single surgeon at a single institution. Ear Nose Throat J. 2005; 9. 84(9):597–602. PMID: 16261761.

7. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 2010. 7th ed. New York, NY: Springer.
8. Hoffman HT, Karnell LH, Robinson RA, Pinkston JA, Menck HR. National Cancer Data Base report on cancer of the head and neck: acinic cell carcinoma. Head Neck. 1999; 7. 21(4):297–309. PMID: 10376748.

9. Spafford PD, Mintz DR, Hay J. Acinic cell carcinoma of the parotid gland: review and management. J Otolaryngol. 1991; 8. 20(4):262–266. PMID: 1920580.
10. Colmenero C, Patron M, Sierra I. Acinic cell carcinoma of the salivary glands: a review of 20 new cases. J Craniomaxillofac Surg. 1991; 8. 19(6):260–266. PMID: 1939673.
11. Batsakis JG, Luna MA, el-Naggar AK. Histopathologic grading of salivary gland neoplasms: II. Acinic cell carcinomas. Ann Otol Rhinol Laryngol. 1990; 11. 99(11):929–933. PMID: 2241022.

12. Ellis GL, Corio RL. Acinic cell adenocarcinoma: a clinicopathologic analysis of 294 cases. Cancer. 1983; 8. 01. 52(3):542–549. PMID: 6861091.

13. Timon CI, Dardick I, Panzarella T, Thomas J, Ellis G, Gullane P. Clinico-pathological predictors of recurrence for acinic cell carcinoma. Clin Otolaryngol Allied Sci. 1995; 10. 20(5):396–401. PMID: 8582068.

14. Jamieson L, Taylor SM, Smith A, Bullock MJ, Davis M. Metastatic acinic cell carcinoma of the parotid gland with ectopic ACTH syndrome. Otolaryngol Head Neck Surg. 2007; 1. 136(1):149–150. PMID: 17210357.

15. Gomez DR, Katabi N, Zhung J, Wolden SL, Zelefsky MJ, Kraus DH, et al. Clinical and pathologic prognostic features in acinic cell carcinoma of the parotid gland. Cancer. 2009; 5. 15. 115(10):2128–2137. PMID: 19309749.

16. Zbaren P, Schupbach J, Nuyens M, Stauffer E, Greiner R, Hausler R. Carcinoma of the parotid gland. Am J Surg. 2003; 7. 186(1):57–62. PMID: 12842751.
17. Stennert E, Kisner D, Jungehuelsing M, Guntinas-Lichius O, Schroder U, Eckel HE, et al. High incidence of lymph node metastasis in major salivary gland cancer. Arch Otolaryngol Head Neck Surg. 2003; 7. 129(7):720–723. PMID: 12874071.

18. Perzin KH, LiVolsi VA. Acinic cell carcinomas arising in salivary glands: a clinicopathologic study. Cancer. 1979; 10. 44(4):1434–1457. PMID: 498020.

19. Lewis JE, Olsen KD, Weiland LH. Acinic cell carcinoma: clinicopathologic review. Cancer. 1991; 1. 01. 67(1):172–179. PMID: 1985714.

20. Spiro RH, Huvos AG, Strong EW. Acinic cell carcinoma of salivary origin: a clinicopathologic study of 67 cases. Cancer. 1978; 3. 41(3):924–935. PMID: 638978.
21. Eneroth CM, Jakobsson PA, Blanck C. Acinic cell carcinoma of the parotid gland. Cancer. 1966; 12. 19(12):1761–1772. PMID: 5927935.

22. Greig SR, Chaplin JM, McIvor NP, Izzard ME, Taylor G, Wee D. Acinic cell carcinoma of the parotid gland: Auckland experience and literature review. ANZ J Surg. 2008; 9. 78(9):754–758. PMID: 18844902.

23. Napier SS, Herron BT, Herron BM. Acinic cell carcinoma in Northern Ireland: a 10-year review. Br J Oral Maxillofac Surg. 1995; 6. 33(3):145–148. PMID: 7654657.

24. Wahlberg P, Anderson H, Biorklund A, Moller T, Perfekt R. Carcinoma of the parotid and submandibular glands: a study of survival in 2465 patients. Oral Oncol. 2002; 10. 38(7):706–713. PMID: 12167424.
25. Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986; Jan-Feb. 8(3):177–184. PMID: 3744850.

26. Chong GC, Beahrs OH, Woolner LB. Surgical management of acinic cell carcinoma of the parotid gland. Surg Gynecol Obstet. 1974; 1. 138(1):65–68. PMID: 4809004.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download