Abstract
Objectives
To investigate the incidence of skin prick test (SPT) positivity in patients with eczematous external otitis.
Methods
Forty-six patients with eczematous external otitis and forty-four healthy volunteers were included in the study. All the patients were skin-tested by prick test. Reactions were assessed by the degree of redness and swelling and the
size of the wheal produced.
Results
According to SPT positivity and total immunoglobulin E values, the difference between the study and the control groups was statistically significant (P<0.05). The most common skin reactions were against to mites and grasses in this study.
Conclusion
Eczematous external otitis is perhaps the most difficult to treat of all forms of external otitis because the provocative agents usually remain undiagnosed. Patients suffering from eczematous external otitis symptoms should be investigated for allergens and be informed for prevention of the causative agents. SPT might be performed in cases of prolonged or treatment-resistant external otitis.
Chronic eczematous external otitis (chronic itchy ears) is a condition characterized by itching, redness, discharge, desquamation, flaking, oozing and, sometimes, fissuring, which are signs and symptoms that suggest inflammation and includes all forms of hypersensitivity of the external ear canal skin. A secondary infection may also occur (1-3).
Common contact allergens include nickel-containing earrings and numerous beauty products (e.g., hairsprays, lotions, hair dye). The relationship between contact allergens and eczematous external otitis has been established (1-3) and aural eczematous dermatitis is more common among people with a predisposition towards atopy and with other similar dermatitides (e.g., seborrhea, psoriasis) (4).
Skin prick testing (SPT) is an allergy test used to identify allergens responsible for triggering symptoms in allergic diseases. While patch testing is a useful diagnostic test for patients with allergic contact dermatitis, SPT is useful in the diagnosis of other allergies such as hay fever and those to food, latex, drugs and bee and wasp venom (5). SPT, with its battery of routine allergens, is still the first and basic procedure in diagnosing allergic diseases. It is simple, carries low risk and is inexpensive to perform (6).
To our knowledge, no previous study has evaluated the relationship between common allergens included in SPT and pure chronic eczematous external otitis in patients with no other associated allergic symptoms. The aim of this study was to investigate the incidence of SPT positivity in patients with eczematous external otitis.
Forty-six patients with eczematous external otitis were included in this study, based on a retrospective chart review at the Rize University Faculty of Medicine, Department of Otorhinolaryngology, Rize, Turkey. Principal symptoms of the patients were itching, pain, discharge and hearing loss for a minimum duration of 3 months. Otomicroscopic examination findings were erythema, desquamation and different degrees of swelling of the external ear canal with no active infection. Patients with perichondritis, fungal infections, perforated eardrums, or infections secondary to otitis media, fronculosis, allergic rhinitis, atopic dermatitis and asthma were excluded. Included patients had no known food allergy except eczematous external otitis. Allergic rhinitis was ruled out by detailed anamnesis, endoscopic evaluation and routine ear/nose/throat examinations. Forty-four age- and sex- matched otherwise healthy patients with previous SPT and venous immunoglobulin E (IgE) sampling were meticulously selected from the outpatient files as the control population. All the patients were skin-tested by a prick test. For at least 3 weeks, none of the patients enrolled in this study took medications, including antihistamines, steroids, topical eardrops, systemic antibiotics, oral corticosteroids and oral and nasal decongestants, since these are liable to affect the skin prick testing. Patients who had active skin disorders or dermatographia were considered to be not suitable for SPT. The tests were performed according to standard methods with allergens, histamine-positive and -negative controls purchased from ALMED (Istanbul, Turkey). Reactions were assessed by the degree of redness and swelling, and the size of the wheal produced. The test was assessed at 20 minutes and the exact size of the wheal was measured and recorded. A growth of 4 mm in diameter in excess of the negative control was considered a positive result. All patients had allergen testing for dust mites Dermatophagoides pteronyssinus, Dermatophagoides farinae, special grass mix 2, special tree mix 1, special mold mix, special weed mix, and mixed epidermals. The control group was determined to be nonallergic as judged by history and routine ear/nose/throat examination. Venous blood samples for total IgE were taken from all cases in the morning after overnight fasting and were determined by a fluoroenzyme immunoassay method.
Findings of the study were statistically assessed using the SPSS ver. 10.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistical methods (frequency) were employed during the evaluation of the study data and Fisher's exact test was used to compare qualitative data. The results obtained from the current study were assessed as within the 95% safety range and the statistical significance was set at P<0.05.
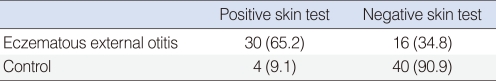
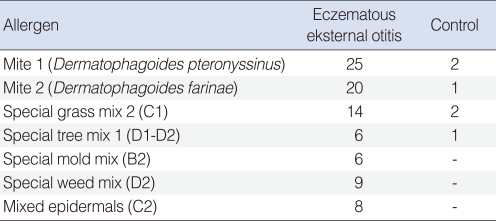
The study group included 46 patients with eczematous external otitis (18 males, 28 females) whose ages ranged between 30 and 72 years. The control group included 44 patients (20 males and 24 females) whose ages ranged between 20 and 64 years. The prick test was positive (wheal size >4 mm) in 30 (65.2%) out of 46 cases with eczematous otitis externa, and 4 (9.1%) out of 44 cases in the control group. SPT results in the two groups are shown in Table 1. According to SPT positivity, the difference between the two groups was statistically significant (P<0.05). Distribution of common allergens is given in Table 2. The most common skin reactions were against mites and grasses. "Total IgE positivity" was defined as total IgE level >165 IU/mL; these levels were elevated in 12 (26.1%) out of 46 cases with eczematous external otitis and no elevation was observed in the control group (Table 3). According to total IgE values, the difference between two groups was statistically significant (P<0.05).
Eczematous external otitis can be an allergic reaction to earrings, eyeglasses, matches, lotions, drugs, shampoo, hair dye, nail polish, earphones or hearing aids and foods, but the frequency of these reactions is not fully known (1, 7). Contact dermatitis requires avoidance or withdrawal of allergic triggers. Trial and error may be needed to identify the offending agent. Topical corticosteroids (e.g., 1% hydrocortisone cream) can decrease inflammation and itching (8). Aural eczematous dermatitis can also be treated with dilute aluminum acetate solution (Burow's solution), which can be applied as often as required for comfort. Itching and inflammation can be reduced with topical corticosteroids. If diffuse external otitis ensues, antibiotic therapy may be required.
Eczematous external otitis tends to be chronic or recurrent (9). Each episode makes the ear more sensitive to future attacks. Chronic changes observed in eczematous external otitis are characterized by thickening of the skin, and even stenosis of the external ear canal may develop. The chronic stage may be bothersome because of periods of uncomfortable itching and the tendency of the patient to resort to scratching, thus causing further irritation (4), leading the irritated skin to a vicious cycle. In the eczematous external otitis, the entire ear canal, concha, intertragic notch and lobule are usually involved. The appearance is that of a confluent mass of weeping, crusted lesions of the hyperemic and edematous skin or even blood clots resulting from forceful scratching (4).
Eczematous external otitis is perhaps the most difficult to treat of all forms of external otitis because the provocative agents usually remain undiagnosed. Allergic contact dermatitis has been diagnosed in 40-58% and 23.5% of patients suffering from otitis externa in two studies (1, 2). Hilen et al. (3) showed that chemical agents (nickel sulfate and inhalants) were the most common allergens, followed by topical drugs. Fraki et al. (10) established that the most common sensitizing agents were topical drugs and the preservatives of topical otic preparations. In some studies, topical drugs were found to be the commonest sensitizing agents. Some studies determined that there was a relationship between food hypersensitivity and eczematous external otitis (4, 11-13), but we could not find any other previous study seeking for the correlation between common system allergens (Dermatophagoides pteronyssinus, Dermatophagoides farinae, special grass mix 2, special tree mix 1, special mold mix, special weed mix, mixed epidermals, etc.) and eczematous external otitis in the English literature. Also, the role of common system allergens tested by SPT was not evaluated before. We considered that common system allergens could have a role in development of eczematous external otitis.
In this retrospective study, SPT positivity in the patients with allergic chronic eczema was diagnosed in 65.2% of the patients suffering from otitis externa. We determined that the commonest allergen group was mites. Total IgE positivity was statistically significant in the study group (26.1%) when compared with the control group.
The present study demonstrated that mite, grass, weed, tree, mold, mixed epidermals hypersensitivity might be a triggering factor, which should be recognized in eczematous external otitis patients. Thus, patients suffering from external otitis symptoms should be assessed for these allergens and allergenic elimination may lead to diminution of current symptoms. Depending on the results of SPT and IgE testings, we can conclude that chronic eczematous external otitis is a subclinical disease without systemic clinical findings (i.e., allergic rhinitis). The most prominent symptom is itching of the ear especially at night, which is due to molds and dust mites usually present where patients sleep. Since allergic contact dermatitis facilitates eczematous external otitis and affects the external auditory canal as a "shock organ", the most important factor in the treatment of all allergic diseases is identifying and removing the present irritant or the allergen. Topical corticosteroids and oral antihistamines as well should be prescribed to break the previously described "vicious cycle". Patients suffering from eczematous external otitis symptoms should be investigated for allergens. SPT might be performed especially in cases of prolonged or treatment-resistant external otitis.
References
1. Yariktas M, Yildirim M, Doner F, Baysal V, Dogru H. Allergic contact dermatitis prevalence in patients with eczematous external otitis. Asian Pac J Allergy Immunol. 2004; 3. 22(1):7–10. PMID: 15366652.
2. Pigatto PD, Bigardi A, Legori A, Altomare G, Troiano L. Allergic contact dermatitis prevalence in patients with otitis externa. Acta Derm Venereol. 1991; 71(2):162–165. PMID: 1675529.
3. Hillen U, Geier J, Goos M. Contact allergies in patients with eczema of the external ear canal: results of the Information Network of Dermatological Clinics and the German Contact Allergy Group. Hautarzt. 2000; 4. 51(4):239–243. PMID: 10810658.
4. Yariktas M, Doner F, Dogru H, Demirci M. Asymptomatic food hypersensitivity prevalence in patients with eczematous external otitis. Am J Otolaryngol. 2004; Jan-Feb. 25(1):1–4. PMID: 15011199.

5. Wantke F, Hemmer W, Jarisch R, Gotz M. Patch test reactions in children, adults and the elderly: a comparative study in patients with suspected allergic contact dermatitis. Contact Dermatitis. 1996; 5. 34(5):316–319. PMID: 8807222.

6. Eigenmann PA, Sampson HA. Interpreting skin prick tests in the evaluation of food allergy in children. Pediatr Allergy Immunol. 1998; 11. 9(4):186–191. PMID: 9920216.

7. Lembo G, Nappa P, Balato N, Pucci V, Ayala F. Contact sensitivity in otitis externa. Contact Dermatitis. 1988; 7. 19(1):64–65. PMID: 3053028.

8. Kristal L, Klein PA. Atopic dermatitis in infants and children: an update. Pediatr Clin North Am. 2000; 8. 47(4):877–895. PMID: 10943263.
9. Sampson HA, Ho DG. Relationship between food-specific IgE concentrations and the risk of positive food challenges in children and adolescents. J Allergy Clin Immunol. 1997; 10. 100(4):444–451. PMID: 9338535.

10. Fraki JE, Kalimo K, Tuohimaa P, Aantaa E. Contact allergy to various components of topical preparations for treatment of external otitis. Acta Otolaryngol. 1985; Nov-Dec. 100(5-6):414–418. PMID: 4082979.
11. Sampson HA. Role of immediate food hypersensitivity in the pathogenesis of atopic dermatitis. J Allergy Clin Immunol. 1983; 5. 71(5):473–480. PMID: 6841827.

13. Woods RK, Thien F, Raven J, Walters EH, Abramson M. Prevalence of food allergies in young adults and their relationship to asthma, nasal allergies, and eczema. Ann Allergy Asthma Immunol. 2002; 2. 88(2):183–189. PMID: 11868923.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download