This article has been
cited by other articles in ScienceCentral.
Abstract
Hypertrophic pachymeningitis is a progressive disease resulting in a diffuse thickening of dura mater due to inflammation, tumor or autoimmune diseases, but most cases are idiopathic. It is seldom reported to be related to sensorineural hearing loss, but it can cause sensorineural hearing loss which can be potentially reversed through treatment. Here, we report the case of a 54-year-old woman who had progressive, bilateral, worse in the left, sensorineural hearing loss and visual disturbance with an accompanying headache over several months. Brain MRI showed diffusely thickened dura mater, highly enhanced after gadolinium administration, which was consistent with pachymeningitis. It was assumed to be related to autoimmune pathogenesis on the basis of elevated serum myeloperoxidase-antineutrophil cytoplasmic antibody (MPO-ANCA) titers. After empirical steroid and cyclophosphamide therapy, auditory impairment improved, especially in the high frequency region of the pure tone audiogram, and significant improvement in the word recognition test. Moreover, a follow-up MRI revealed much decreased enhancement of the dura mater, and the MPO-ANCA titer decreased to within the normal range. In the case of rapidly progressive sensorineural hearing loss or hearing impairment accompanying other cranial neuropathy, pachymeningitis should be taken into consideration, and brain MRI with gadolinium enhancement is the best method of detecting it. Also, to ensure proper treatment, a cautious evaluation including an ANCA work-up should be performed.
Go to :

Keywords: Hypertrophic pachymeningitis, Multiple cranial neuropathies, p-ANCA, Wegener's granulomatosis
INTRODUCTION
Sensorineural hearing loss can have various causes, and it is mostly irreversible. However, it can be managed with treatment in some instances such as autoimmune hearing loss (
1,
2). Therefore, in cases of atypical sensorineural hearing loss, such as hearing loss that progresses relatively rapidly, or when neurologic signs coexist, the possibility of reversible causes should not be ruled out. Also, in order to perform a proper evaluation, otologists should be aware of the potential causes of reversible sensorineural hearing loss.
Pachymeningitis is an uncommon disorder characterized by localized or diffuse thickening of the dura mater, and is rarely reported to lead to sensorineural hearing loss (
3-
6). Even though pachymeningitis is a potentially treatable cause of sensorineural hearing loss (
4-
6), it is quite unfamiliar to otologists and difficult to diagnose.
Here, we present the case of a patient who had rapidly progressive, bilateral sensorineural hearing loss due to hypertrophic pachymeningitis. It was associated with high serum levels of myeloperoxidase-antineutrophil cytoplasmic antibody (MPO-ANCA).
Go to :

CASE REPORT
A 54-year-old woman presented with a progressive visual disturbance and hearing impairment, especially in the left ear, over the previous month. She also complained of recent intensification of a throbbing headache in her left fronto-temporal area that had been present for 6 months.
An audiologic test revealed bilateral, down-sloping, moderate, sensorineural hearing loss with a mean threshold of 50 dB in the pure tone audiogram (
Fig. 1A). Word recognition scores were 100% in the right ear and 24% in the left. Ophthalmic examination showed both peripapillary edema and atrophy, which was consistent with optic neuropathy. Other neurologic examinations yielded normal findings.
 | Fig. 1Pure tone audiograms and word recognition scores before and after treatment. Initial pure tone audiogram (A). Pure tone audiogram after a 4-month period of steroid and cyclophosphamide treatment. Hearing improvement was detected, especially in the high frequency region (arrow) (B). Serially checked word recognition scores in the left ear after steroid and cyclophosphamide treatment (C). Note that the word recognition score went up daily after steroid administration and in month 4 it reached 100%. On the other hand, the word recognition score in the right ear was 100% in every test. 
|
Laboratory tests showed an elevated erythrocyte sedimentation rate (ESR) to 47 mm/hour (normal range, 0 to 20 mm/hour), a C-reactive protein (CRP) level up to 4.66 mg/dL (normal range<0.8 mg/dL), and normal cerebrospinal fluid (CSF).
Brain magnetic resonance imaging (MRI) revealed diffuse thickening of meninges which appeared hypodense and isointense on T1 and T2 weighted images (
Fig. 2). The meninges were markedly enhanced after gadolinium administration (
Fig. 3A), which was consistent with hypertrophic pachymeningitis.
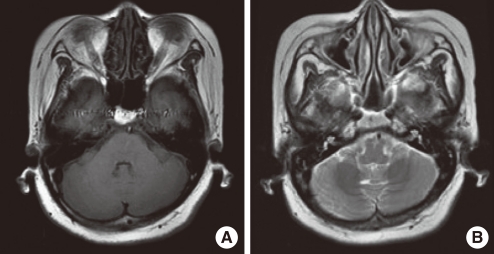 | Fig. 2Initial brain MRI. Axial T1-weighted image shows hypo-intense thickened dura mater (A). Axial T2-weighted image shows isointense dura mater with hyperintense border (B). 
|
 | Fig. 3The gadolinium enhanced T1-weighted axial and coronal images. Initial images. They demonstrate diffusely enhanced thick dura mater around cerebellopontine angle (arrow) (A). After 4-month steroid and cyclophosphamide treatment (B). After 9-month steroid and cyclophosphamide maintenance (C). Note that diseased dura mater (arrow) became less thickened compared with initial MR image as treatment was maintained. 
|
A chest X-ray and chest computed tomography showed no evidence of silicosis or sarcoidosis or recent tuberculosis. Positron emission tomography-computed tomography (PET-CT) and tests for tumor markers were unremarkable. Upon immunological investigation, autoimmune antibodies were found to be in the normal range, except for an elevated serum MPO-ANCA (an autoantibody titer [AAU] of 388; normal range, 0 to 150 AAU). A diagnosis of hypertrophic pachymeningitis associated with a high serum level of MPO-ANCA was made. Treatment was started empirically with high dose steroids (1 mg/kg) and cyclophosphamide (Alkyloxan, Choong Wae Pharm, Seoul, Korea) 1.5 mg/kg. During the administration of steroids and cyclophosphamide, her headache gradually subsided.
Over a 4-month treatment period with steroids and cyclophosphamide, visual loss and hearing impairment gradually improved. On repeat audiologic testing, the pure tone threshold was partially improved when compared with the initial pure tone audiogram, especially in the high frequency region (
Fig. 1B) and there were much improved results in word recognition scores (
Fig. 1C). Also, MRI showed less thickened dura mater and diffuse regression of dural enhancement (
Fig. 3B, C). The MPO-ANCA titer gradually decreased to be within the normal range (normal range, 0 to 150 AAU) (
Fig. 4).
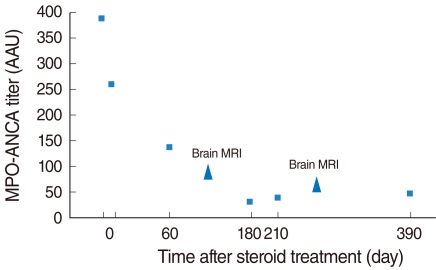 | Fig. 4Changes in myeloperoxidase-antineutrophil cytoplasmic antibody (MPO-ANCA) titer during steroid and cyclophosphamide treatment. Note that the initial high titer of MPO-ANCA (388AAU) decreased to within the normal range as treatment was maintained. Brain MRI scans were done at 4 and 9 months after treatment. 
|
So far, the patient has been on steroids and cyclophosphamide at a maintenance dose (steroid 10 mg; Alkyloxan 50 mg per day). During the follow up period of 1 year, her symptoms remained well-controlled and there was no evidence of recurrence of the problem according to serial MRI scans and MPO-ANCA titers.
Go to :

DISCUSSION
Hypertrophic pachymeningitis is a progressive disease resulting in a diffuse thickening of the dura mater. It is caused by inflammation, tumor or autoimmune diseases such as tuberculosis, syphilis, sarcoidosis, Wegener's granulomatosis, and rheumatoid arthritis, but mostly it is idiopathic (
3,
7). Recently, more research on the relationship between pachymeningitis and autoimmune pathogenesis has been carried out. Moreover, there have been several reports about pachymeningitis with autoimmune antibody. In particular, the relationship between ANCA-positive pachymeningitis and vasculitis has been discussed (
8-
10). In our case, MPO-ANCA related autoimmune pathogenesis caused thickening of the dura mater near the tentorium cerebella.
ANCAs have been classified as cytoplasmic (c-ANCA) or perinuclear (p-ANCA) based on their immunofluorescence patterns. The antigens responsible for these patterns are proteinase 3 (PR3) for c-ANCA and MPO for p-ANCA (
11). Since Falk and Jennette (
8) first identified MPO-ANCA, it has been used for the diagnosis of vasculitis-associated disease such as Churg-Strauss syndrome and microscopic polyangitis (
12). Also, it was reported that the titer of MPO-ANCA decreases as the disease gradually improves through immunosuppressive treatment. Hence, it is suggested that an MPO-ANCA titer can be used as a monitoring marker of the disease's activity in such patients (
12). In this case, the thickness of the meninges and MPO-ANCA titer corresponded relatively well, so her MPO-ANCA titer was checked regularly to monitor the disease.
To diagnose pachymeningitis, MRI with gadolinium enhancement is the most sensitive diagnostic imaging modality, and shows the relationship between the inflammatory degree of the lesion and the clinical outcome, as well as the prognosis (
3,
7). In MR imaging, T1-weighted images show iso- or hypo-intense thickened dura mater, and T2-weighted images show hypointense dura mater of fibrous reacting tissue surrounded by a thin hyperintense border attributed to the acute inflammatory process. Distinctively, meninges are highly enhanced after a contrast injection (
7).
In pachymeningitis, cranial nerve involvement is of concern due to high intracranial pressure resulting from thickened dura mater; or it may be directly caused by small-vessel vasculitis or indirectly by the inflammatory process of the surrounding soft tissue or of the meninges? (
13). In most cases, optic nerve involvement is reported along with headache (
3,
7,
14), but 8th nerve involvement is seldom reported (
4-
6).
Our patient showed auditory impairment, which was unusual. Generally in ANCA-associated vasculitis, it is assumed that small vessels are impaired in the inner ear, which causes sensorineural hearing loss bilaterally (
1). However, in this case, the patient complained of unilateral hearing impairment, and the word recognition score in that side was poorer than expected. Several studies show that ordinary tone and speech audiometry yields little diagnostic information about lesions (
15-
17). However, retrocochlear pathology would be suspected in the presence of a significant discrepancy in word recognition score between two ears or a lower than expected performance in speech audiometry (
17-
19). In this patient, after steroid treatment, the pure tone threshold at three consecutive frequencies (3,000, 4,000, 8,000 Hz) improved by 28 dB in the left and 16 dB in the right ear, and the word discrimination score improved from 26% to 100% in the left. Considering that there was a poor word recognition score on the ipsilateral side and that its recovery was consistent with improvement of the meninges, we suspected that a retrocochlear lesion such as fibrous entrapment of auditory nerve due to thickened dura mater was involved as well as a cochlear lesion due to autoimmune processes.
In conclusion, pachymeningitis may cause sensorineural hearing loss that is sometimes reversible. To make an early diagnosis, gadolinium enhanced MR imaging and the otologists' awareness of pachymeningitis are important. Moreover, for proper treatment, the causes of pachymeningits should be evaluated, including a serologic test, autoimmune test and imaging. In the case of atypical sensorineural hearing loss accompanied by multiple cranial nerve palsy or headache, pachymeningitis should be considered in the differential diagnosis.
Go to :









 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download