Abstract
Objectives
Carboplatin, a platinum-containing anti-cancer drug used to treat a variety of cancers, induces ototoxicity. Since, reactive oxygen species (ROS) and nitric oxide (NO) seem to be responsible for this toxicity, the antioxidant, N-acetyl-L-cysteine (L-NAC), and NO synthetase inhibitor, N-nitro-L-arginine methyl ester (L-NAME) were predicted to have protective effects against carboplatin ototoxicity. The aim of this study was to test for the protective effects of L-NAC and L-NAME on cochlear hair cells and spiral ganglion neurons (SGNs).
Methods
Cochlear organotypic cultures and dissociated spiral ganglion neuron cultures, from mice postnatal day 5 cultures were used in this study. The cultures were treated with carboplatin alone or in combination with L-NAC or L-NAME, and carboplatin-induced damage was monitored.
Results
Treatment with carboplatin induced a significant loss of outer hair cells, while inner hair cells were preserved in the cochlear organotypic cultures. Addition of L-NAC or L-NAME reduced the amount of carboplatin-induced hair cell damage; the differences did not reach statistical significance. However, carboplatin significantly decreased the number of surviving SGNs in dissociated cultures. The toxic effects were significantly reduced by addition of L-NAC or L-NAME. In addition, carboplatin induced the loss of neurites from the SGN somata, and this was not blocked with L-NAC or L-NAME.
Carboplatin (cis-diammine [1,1-cyclobutanedicarboxylato]-platinum [II]) is a second generation platinum-containing anti-cancer drug used for the treatment of patients with solid tumors such as head and neck, lung and ovarian carcinomas (1-3). Although carboplatin has fewer toxic effects than cisplatin, the clinical use of carboplatin can be complicated by nephrotoxicity, neurotoxicity and ototoxicity (4). Permanent sensorineural hearing loss, particularly at high frequencies, has been reported among patients treated with carboplatin (5, 6).
Though several studies have sought to determine the underlying mechanism leading to the adverse neurotoxic effects of carboplatin, the responsible mechanisms leading to carboplatin ototoxicity are not fully understood. However, several lines of evidence suggest that damage may be related to an enhanced flux of reactive oxygen species (ROS) and reactive nitrogen species (RNS) (7-9). Both ROS and RNS, as potential neurotoxins, may be generated by carboplatin and could contribute significantly to the damage and death of hair cells and spiral ganglion neurons (SGNs). To date, few reports have focused on the protective effects of antioxidants or nitric oxide synthetase (NOS) on carboplatin-induced ototoxicity.
N-acetyl-L-cysteine (L-NAC) is a strong antioxidant and induces de novo synthesis of glutathione (GSH). GSH plays a central role in the GSH redox cycle, which is important in neutralizing free radicals and providing protection from free hyperperoxides and lipid peroxides (10). Administration of L-NAC has been shown to provide significant protection against oxidative stress caused by the depletion of cellular antioxidants and the generation of ROS and free radicals in noise-induced hearing loss (11). In addition, N-nitro-L-arginine methyl ester (L-NAME), an L-arginine analog, non-selectively competes with L-arginine and inhibits many different NOS. A previous study reported that NO production decreased when the perilymph was perfused with L-NAME in a noise-induced hearing loss model (12). Similarly, noise-induced increase in the NO levels in the cochlea was significantly attenuated by L-NAME in another study (13).
In this study, carboplatin induced losses of hair cell and SGN were evaluated in cochlear organotypic and dissociated SGN cultures. In addition, whether treatment with L-NAC and L-NAME has a protective effect against carboplatin-induced ototoxicity was studied.
Cochleae were dissected from postnatal day 5 (P5) mouse pups under the microscope. The skull was opened and the temporal bones were harvested. The cochleae containing the middle turn of the organ of Corti were removed from the temporal bone and cultured for 24 hours in Dulbecco's Modified Eagle Medium (DMEM; Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; Invitrogen). These tissues were cultured in collagen-coated Transwells (Corning Life Science, Corning, NY, USA). During the initial 24-hour culture, the cochleae of the L-NAC (Sigma, St. Louis, MO, USA) and L-NAME (Sigma) groups were placed in DMEM containing 10% FBS added with L-NAC (10 mM) and L-NAME (100 mM), respectively. Thereafter, the cochleae were placed in different media according to the group as described below. The cochleae from the control group were placed in DMEM containing 10% FBS for additional 48 hours, while the cochleae from the carboplatin , L-NAC and L-NAME groups were placed in media with carboplatin added (100 mg/mL), carboplatin plus L-NAC (10 mM), and carboplatin plus L-NAME (100 mM), respectively, for 48 hours.
Dissociated SGNs cultures were prepared from P5 mice cochleae using a procedure described previously (14). Cochleae were aseptically removed from the temporal bone and placed in ice-cold phosphate buffered saline (PBS). The bony cochlear capsule and spiral ligament were removed. The organ of Corti was then removed, transecting the outer radial fibers, leaving the SGNs within the modiolus. Modiolar bone was removed and surrounding connective tissue was incompletely removed. Ganglia were collected in ice-cold Hank's balanced salt solution (HBSS).
Enzymatic dissociation was performed in Ca2+/Mg2+-free HBSS with 0.1% collagenase, 0.1% trypsin, and 0.01% DNase I (Boehringer Mannheim, Indianapolis, IN, USA) in a gently shaking 37℃ water bath for 30 minutes. FBS was added to 10% to inhibit enzymatic activity, followed by three washes in serum-free DMEM. The ganglia were dissociated mechanically using two fire-polished reduced orifice glass pipettes. The second pipette used in this procedure was considerably narrower than the first. The ganglia were gently triturated about 15 times with each pipette and filtered through a nylon mesh (Nitex, 15 mm, Tetko Inc., Elmsford, NY, USA).
Dissociated spiral ganglion cells were plated in 8-well slides (Marienfeld, Bad Mergentheim, Germany) that had been treated with 0.01% polyornithin (Sigma) for one hour at room temperature, followed by laminin (20 µg/mL; Sigma) overnight at 4℃. The dissociated cells were cultured in 100 µL of culture media (N2, 0.5×B27 and insulin supplemented high-glucose DMEM) at 37℃ in a 5% CO2 incubator. The controls were treated with 50 ng/mL BDNF (Invitrogen) and 50 ng/mL NT-3 (Invitrogen). After an initial 24-hour culture, the cells were exposed to carboplatin (50 mg/mL) for an additional 48 hours. The cells in the antioxidant-treatment groups were exposed to L-NAC (100 mM), L-NAME (100 mM) and L-NAC plus L-NAME during both the initial 24-hours culture period and the additional 48-hours of carboplatin treatment. All in vitro experiments were repeated three times and similar results were obtained each time.
The cultured tissues were fixed in 4% paraformaldehyde and washed three times with PBS. The specimens were then reacted with 0.1% Triton X-100 for 15 minutes at room temperature. After washing, the specimens were reacted with a blocking serum (normal goat serum) for one hour and incubated overnight with a 1:400 dilution of the anti-NF 200 antibody (Sigma). The specimens were then washed and incubated with a 1:50 dilution of rhodamine-conjugated IgG (Zymed, South San Francisco, CA, USA) for 30 minutes at 37℃. Then, the tissues were incubated with FITC-labeled phalloidin (0.1 mg/mL) for 20 minutes at room temperature.
The staining procedures of dissociated SGN cultures were performed in the same manner as the tissues, except using different secondary conjugated fluorescent dye (FITC-conjugated IgG; Zymed). For nuclear staining, Hoecsht 33258 (1 mg/mL; Invitrogen) was used. The specimens were finally washed and mounted for microscopic examination. The specimens were examined under a confocal and fluorescence microscope. The criteria used to determine neuronal viability were 1) NF-200 positivity, with a visible nucleus, and 2) absence of nuclear pyknosis.
Fig. 1A shows the typical arrangement of inner hair cells (IHCs) and outer hair cells (OHCs) in untreated 72 hour cochlear organotypic cultures. The neurites from the SGN somata were well visualized. Although the structure of the organ of Corti was maintained in the carboplatin-treated group, most of the stereocilia bundles of OHCs were slightly destroyed (Fig. 1B). A few of the OHCs in the third row were missing while a considerable number of them were present in the first and second rows. Stereocilia in IHCs also showed minimal deformations. In addition, some of the neurite connections between hair cells and SGNs, in the carboplatin-treated group, were missing or extremely thin and difficult to discern compared to those in the control group. However, these destructive changes of the hair cells in the organ of Corti were not definite in both groups pre-treated with L-NAC and L-NAME (Fig. 1C, D). The damaged neurite connections after carboplatin treatment were not recovered by addition of L-NAC or L-NAME.
Fig. 2 shows the mean number of IHCs and OHCs in the middle turn of the cochleae in each group. No differences were observed among all experimental groups regarding the number of IHCs (Fig. 2A). The number of OHCs in the carboplatin-treated group was significantly lower than in the control group (P=0.025). However, slight differences were noted between the number of OHCs in the carboplatin-treated group and those in L-NAME/L-NAC treated groups, even though the differences did not reach statistical significance (Fig. 2B).
SGNs were stained with NF-200, a neuronal specific marker, and neuronal survival was determined by counting NF-200-positive cells as described in the Materials and Methods section. As shown in Fig. 3, the dissociated SGNs treated with carboplatin showed cytotoxicity (Fig. 3B) compared to the untreated group (Fig. 3A). As shown in Fig. 3C and D, L-NAME and L-NAC promoted survival compared to the control group. In addition, the cytotoxicity of the SGNs in the carboplatin-treated group was partially prevented when L-NAME or L-NAC were added prior to carboplatin administration; however, neurite outgrowth was not affected by the addition of L-NAME or L-NAC (Fig. 3E, F).
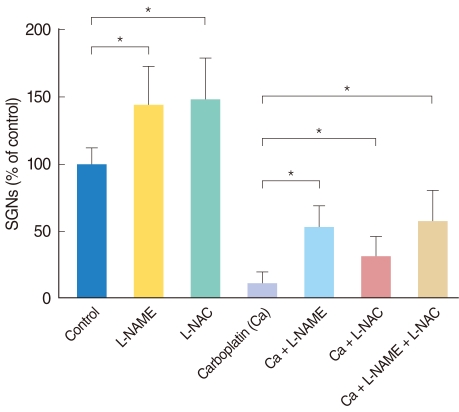
Neuronal viability in the experimental conditions is expressed as the percentage of the average number of NF-200-positive cells in triplicate parallel cultures in the control group (Fig. 4). The number of surviving SGNs, in the groups treated with either L-NAME or L-NAC alone, was significantly higher than in the control group. Administration of carboplatin significantly reduced the mean number of surviving SGNs in dissociated cultures compared to the control group. Addition of L-NAME or L-NAC appeared to rescue SGNs from carboplatin-induced death; these differences were statistically significant. There was no significant difference in the mean number of surviving SGNs between the groups treated with L-NAME and L-NAC. Moreover, the combination of L-NAME plus L-NAC did not show an additive effect on neuronal survival compared to treatment with either agent alone.
Carboplatin is currently used in oncology clinics for the treatment of a variety of cancers. It is considered an alternative anticancer drug, and usually recommended for chemotherapy in patients with ovarian cancer, lung cancer and head and neck cancer. High dose carboplatin has been shown to cause hearing loss as a side effect in cancer patients (15). Therefore, to clarify the mechanism of carboplatin-induced ototoxicity, many studies have been performed with various animal models.
The ototoxic mechanism associated with carboplatin treatment remains unknown. However, the available evidence implies that carboplatin-induced ototoxicity results from enhanced flux of free radicals and depletion of antioxidants in the cochlea (7, 9). In addition, a recent study revealed that cytotoxicity induced by cisplatin, which is an analogue of carboplatin, is associated with an increase in ROS production (16). ROS has known to play a major role in loss of outer hair cells and spiral ganglia of mouse after cisplatin administration (17). Furthermore, NO is a simple inorganic radical that is produced by different isoforms of a nicotinamide adenine dinucleotide phosphate hydrogen (NADPH)-dependent enzyme, NOS, and known to be associated with cochlear pathology in a variety of conditions (12, 18, 19).
An earlier study showed that carboplatin significantly increased NO levels and decreased GSH peroxidase levels in the cochleae of rats (7). In addition, hearing thresholds were changed by NO elevation and GSH depletion in the cochleae of rats treated with carboplatin. A recent study also reported a significant role of NO in carboplatin-induced ototoxicity (9). Cochlear NO levels were significantly higher in rats treated with carboplatin than in the controls. Moreover, administration of L-NAC prior to carboplatin treatment decreased NO levels in the cochleae and had a protective effect against carboplatin-induced ototoxicity. Furthermore, similar results were observed in mouse that apoptosis triggered by inducible NOS mediates the ototoxicity induced by cisplatin, which has similar characteristics of carboplatin (20). The results of this study are consistent with the findings of previous studies.
In the present study, we demonstrated that carboplatin induced significant OHC damage, especially in the third row, in cochlear organotypic cultures. By contrast, IHC loss was not definite; there was no difference in the number of IHCs between the control and treated groups. However, minimal deformation of the stereocilia in the IHCs was observed. Cochlear organotypic cultures were obtained from P5 mice; therefore, this predilection of injury in the OHCs may be a characteristic of these newborn mice. In cochlear organotypic cultures, L-NAME or L-NAC administration did not have a statistically significant protective effect on hair cells. However, there was an increasing trend of survival of the OHCs after administration of L-NAME or L-NAC; however, the changes did not reach statistical significance.
In order to clarify the effect of carboplatin on SGNs, carboplatin was added to dissociated SGN cultures. The mean number of surviving SGNs, in the groups with L-NAME or L-NAC without carboplatin treatment, was significantly higher than in the control group. These results imply that L-NAME or L-NAC themselves are effective for the survival of SGNs in dissociated cultures. These compounds may attenuate the toxic effects of ROS or RNS naturally generated in the normal culture environment. Free radicals and NO may be responsible for spontaneous SGN death in the culture medium.
In this study, carboplatin treatment significantly reduced the mean number of surviving SGNs in dissociated cultures. A previous study also demonstrated a distinct loss of SGNs after carboplatin administration in a chinchilla model (21). However, in the previous study, the SGN population decreased progressively from 2 to 12 weeks after IHC loss. Therefore, they postulated that selective damage to the IHCs caused SGN loss. By contrast, the results of this study demonstrated direct cytotoxicity to the SGNs in dissociated cultures. Interspecies differences to the susceptibility to carboplatin might explain the difference between these two studies.
Administration of carboplatin might have an adverse effect on neurite growth from the SGN somata in dissociated cultures, as observed in this study. This finding is consistent with the findings of a previous study that reported the inhibition of neurite outgrowth in organotypic cultures of rat dorsal root ganglia treated with carboplatin (22). However, the mechanism responsible for this inhibition is not fully understood. Administration of L-NAME or L-NAC did not attenuate the toxic effects of carboplatin on neurites in this study. The results of a recent study showed that the physiological level of the ROS was critical for maintaining and controlling neurite outgrowth in the neuronal cells (23). Moreover, it has been reported that NO plays a critical role in neurite outgrowth of neuroblastoma cells and that the addition of L-NAME inhibits neurite outgrowth of neuroblastoma cells (24). However, L-NAME or L-NAC alone did not affect the neurite length of damaged SGNs by carboplatin while they promoted the survival of SGNs in this study. Therefore, ROS and RNS may not be involved in the inhibition of neurite outgrowth of SGNs in mice exposed to carboplatin.
In addition, pre-administration of L-NAME and L-NAC significantly attenuated carboplatin-induced cytotoxicity in dissociated SGN cultures; however, they did not show an additive effect. The direct toxicity of NO is known to be modest but is enhanced by reactions with superoxide to form peroxynitrite, which has direct cytotoxicity (25). Thus, NO and the related ROS may cause cell death in an additive manner. However, combination of L-NAME and L-NAC did not have an additive effect on neuronal survival compared to treatment with either agent alone, in this study. It is postulated that the mechanism, related to ROS and RNS, might share an overlapping pathway leading to damage and death in carboplatin-induced cytotoxicity in dissociated SGN cultures.
Carboplatin-induced ototoxicity has been demonstrated in a variety of experimental animals (9, 26-29). A number of previous studies showed that ototoxicity to carboplatin varies considerably across different species. IHCs and type-I spiral ganglion neurons are preferentially destroyed in carboplatin-treated chinchillas, while OHCs are spared (26). Guinea pigs are relatively resistant to the ototoxic effects of carboplatin. However, when ototoxicity develops after treatment with carboplatin in guinea pigs, selective degeneration of the OHCs near the base of the cochlea occurs and the IHCs remain intact (29, 30). Although many animal studies have been performed to elucidate the mechanism of carboplatin-induced ototoxicity, there have been few prior reports using mice. Therefore, the feasibility of a mouse model for carboplatin-induced ototoxicity was examined in this study (data not shown).
Fifteen 5-6 week C57BL/6J mice were used in this study. Unexpectedly, 12 (80%) out of 15 mice treated with carboplatin (120 mg/kg, intraperitoneal injection) died within 2 weeks, and the remaining three mice were included in the analysis. This unanticipated result may be due to different individual susceptibilities to carboplatin among the mice. It is postulated that nephrotoxicity may precede ototoxicity in a mouse model. The byproduct of carboplatin, platinum-ammine-DNA adduct, accumulates in the kidney and induces renal tubular damage (31). This accumulation of byproducts might be responsible for the death of the mice in this study. All three surviving mice showed significant cochlear damage, including partial-to-total loss of OHCs and minimal deformation of IHCs, which are consistent with the results from the organotypic cultures in this study. Although significant damage was identified in some mice in this study, a high proportion of death and different individual susceptibilities suggest that a mouse model is not suitable for experiments studying carboplatin-induced ototoxicity.
In summary, carboplatin is toxic to cochlear hair cells, SGNs and neurites between hair cells and SGNs in organotypic and dissociated cultures from mice. In addition, administration of L-NAME and/or L-NAC attenuated cytotoxicity of carboplatin to SGNs in dissociated SGN cultures. Therefore, reactive oxygen radicals and NO may be responsible for certain damage to the cochlea caused by carboplatin treatment.
ACKNOWLEDGMENT
This work was supported by grant No. 10031764 from the Strategic Technology Development of Ministry of Knowledge Economy of Korea.
References
1. Ahmed SM, Cohen EE. Treatment of squamous cell carcinoma of the head and neck in the metastatic and refractory settings: advances in chemotherapy and the emergence of small molecule epidermal growth factor receptor kinase inhibitors. Curr Cancer Drug Targets. 2007; 11. 7(7):666–673. PMID: 18045071.

2. Goffin J, Lacchetti C, Ellis PM, Ung YC, Evans WK. Lung Cancer Disease Site Group of Cancer Care Ontarios Program in Evidence-Based Care. First-line systemic chemotherapy in the treatment of advanced non-small cell lung cancer: a systematic review. J Thorac Oncol. 2010; 2. 5(2):260–274. PMID: 20101151.

3. Marchetti C, Pisano C, Facchini G, Bruni GS, Magazzino FP, Losito S, et al. First-line treatment of advanced ovarian cancer: current research and perspectives. Expert Rev Anticancer Ther. 2010; 1. 10(1):47–60. PMID: 20014885.

4. Esteban-Fernandez D, Verdaguer JM, Ramirez-Camacho R, Palacios MA, Gomez-Gomez MM. Accumulation, fractionation, and analysis of platinum in toxicologically affected tissues after cisplatin, oxaliplatin, and carboplatin administration. J Anal Toxicol. 2008; 3. 32(2):140–146. PMID: 18334097.

5. Crepaldi de Almeida EO, Umeoka WG, Viera RC, de Moraes IF. High frequency audiometric study in cancer-cured patients treated with cisplatin. Braz J Otorhinolaryngol. 2008; May–Jun. 74(3):382–390. PMID: 18661012.
6. Dean JB, Hayashi SS, Albert CM, King AA, Karzon R, Hayashi RJ. Hearing loss in pediatric oncology patients receiving carboplatin-containing regimens. J Pediatr Hematol Oncol. 2008; 2. 30(2):130–134. PMID: 18376265.

7. Husain K, Whitworth C, Somani SM, Rybak LP. Carboplatin-induced oxidative stress in rat cochlea. Hear Res. 2001; 9. 159(1-2):14–22. PMID: 11520631.

8. Kelly TC, Whitworth CA, Husain K, Rybak LP. Aminoguanidine reduces cisplatin ototoxicity. Hear Res. 2003; 12. 186(1-2):10–16. PMID: 14644455.

9. Okur E, Kilinc M, Yildirim I, Kilic MA, Tolun FI. Effect of N-acetyl-cysteine on carboplatin-induced ototoxicity and nitric oxide levels in a rat model. Laryngoscope. 2007; 12. 117(12):2183–2186. PMID: 17909450.
10. Rahman Q, Abidi P, Afaq F, Schiffmann D, Mossman BT, Kamp DW, et al. Glutathione redox system in oxidative lung injury. Crit Rev Toxicol. 1999; 11. 29(6):543–568. PMID: 10628776.

11. Coleman JK, Kopke RD, Liu J, Ge X, Harper EA, Jones GE, et al. Pharmacological rescue of noise induced hearing loss using N-acetylcysteine and acetyl-L-carnitine. Hear Res. 2007; 4. 226(1-2):104–113. PMID: 17023129.

12. Shi X, Ren T, Nuttall AL. The electrochemical and fluorescence detection of nitric oxide in the cochlea and its increase following loud sound. Hear Res. 2002; 2. 164(1-2):49–58. PMID: 11950524.

13. Diao M, Gao W, Sun J. Nitric oxide synthase inhibitor reduces noise-induced cochlear damage in guinea pigs. Acta Otolaryngol. 2007; 11. 127(11):1162–1167. PMID: 17851886.

14. Hegarty JL, Kay AR, Green SH. Trophic support of cultured spiral ganglion neurons by depolarization exceeds and is additive with that by neurotrophins or cAMP and requires elevation of [Ca2+]i within a set range. J Neurosci. 1997; 3. 15. 17(6):1959–1970. PMID: 9045725.
15. Kushner BH, Budnick A, Kramer K, Modak S, Cheung NK. Ototoxicity from high-dose use of platinum compounds in patients with neuroblastoma. Cancer. 2006; 7. 15. 107(2):417–422. PMID: 16779793.

16. Kim HJ, Lee JH, Kim SJ, Oh GS, Moon HD, Kwon KB, et al. Roles of NADPH oxidases in cisplatin-induced reactive oxygen species generation and ototoxicity. J Neurosci. 2010; 3. 17. 30(11):3933–3946. PMID: 20237264.

17. Lee JE, Nakagawa T, Kim TS, Endo T, Shiga A, Iguchi F, et al. Role of reactive radicals in degeneration of the auditory system of mice following cisplatin treatment. Acta Otolaryngol. 2004; 12. 124(10):1131–1135. PMID: 15768804.

18. Li G, Liu W, Frenz D. Cisplatin ototoxicity to the rat inner ear: a role for HMG1 and iNOS. Neurotoxicology. 2006; 1. 27(1):22–30. PMID: 16125245.
19. Yamane H, Takayama M, Sunami K, Iguchi H, Kanazawa A, Tokuhara Y, et al. Nitric oxide induces apoptosis of the hair cells of cochlea. Acta Otolaryngol Suppl. 2004; 10. (554):6–11. PMID: 15513503.

20. Watanabe K, Inai S, Jinnouchi K, Bada S, Hess A, Michel O, et al. Nuclear-factor kappa B (NF-kappa B)-inducible nitric oxide synthase (iNOS/NOS II) pathway damages the stria vascularis in cisplatin-treated mice. Anticancer Res. 2002; Nov–Dec. 22(6C):4081–4085. PMID: 12553036.
21. Takeno S, Wake M, Mount RJ, Harrison RV. Degeneration of spiral ganglion cells in the chinchilla after inner hair cell loss induced by carboplatin. Audiol Neurootol. 1998; Sep–Oct. 3(5):281–290. PMID: 9705525.
22. Jirsova K, Mandys V. Differences in the inhibition of neuritic outgrowth in organotypic cultures of rat foetal dorsal root ganglia treated with cisplatin and carboplatin: a comparative study. Folia Histochem Cytobiol. 1997; 35(4):215–219. PMID: 9619421.
23. Munnamalai V, Suter DM. Reactive oxygen species regulate F-actin dynamics in neuronal growth cones and neurite outgrowth. J Neurochem. 2009; 2. 108(3):644–661. PMID: 19054285.

24. Evangelopoulos ME, Wüller S, Weis J, Krüttgen A. A role of nitric oxide in neurite outgrowth of neuroblastoma cells triggered by mevastatin or serum reduction. Neurosci Lett. 2010; 1. 01. 468(1):28–33. PMID: 19853642.

25. Ohinata Y, Miller JM, Schacht J. Protection from noise-induced lipid peroxidation and hair cell loss in the cochlea. Brain Res. 2003; 3. 21. 966(2):265–273. PMID: 12618349.

26. Ding DL, Wang J, Salvi R, Henderson D, Hu BH, McFadden SL, et al. Selective loss of inner hair cells and type-I ganglion neurons in carboplatin-treated chinchillas. Mechanisms of damage and protection. Ann N Y Acad Sci. 1999; 11. 28. 884:152–170. PMID: 10842592.

27. Taudy M, Syka J, Popelár J, Ulehlová L. Carboplatin and cisplatin ototoxicity in guinea pigs. Audiology. 1992; 31(5):293–299. PMID: 1449432.
28. Wake M, Takeno S, Ibrahim D, Harrison R, Mount R. Carboplatin ototoxicity: an animal model. J Laryngol Otol. 1993; 7. 107(7):585–589. PMID: 15125271.

29. Watanabe KC, Jinnouchi K, Hess A, Michel O, Baba S, Yagi T. Carboplatin induces less apoptosis in the cochlea of guinea pigs than cisplatin. Chemotherapy. 2002; 5. 48(2):82–87. PMID: 12011540.

30. Saito T, Saito H, Saito K, Wakui S, Manabe Y, Tsuda G. Ototoxicity of carboplatin in guinea pigs. Auris Nasus Larynx. 1989; 16(1):13–21. PMID: 2475098.

31. Kintzel PE. Anticancer drug-induced kidney disorders. Drug Saf. 2001; 1. 24(1):19–38. PMID: 11219485.

Fig. 1
Confocal microscopic photomicrographs of cochlear organotypic cultures stained with FITC-conjugated phalloidin (green) and NF-200 (red). (A) Control showing a typical arrangement of three rows of outer hair cells, and single row of inner hair cells. (B) Cochlear cultures treated for 48 hours with carboplatin at a concentration of 100 mg/mL. (C) Cochlear cultures treated for 48 hours with 100 µg/mL carboplatin plus 100 µM N-nitro-L-arginine methyl ester. (D) Cochlear cultures treated for 48 hours with 100 µg/mL carboplatin plus 10 mM N-acetyl-L-cysteine. Scale bar represents 10 µm.
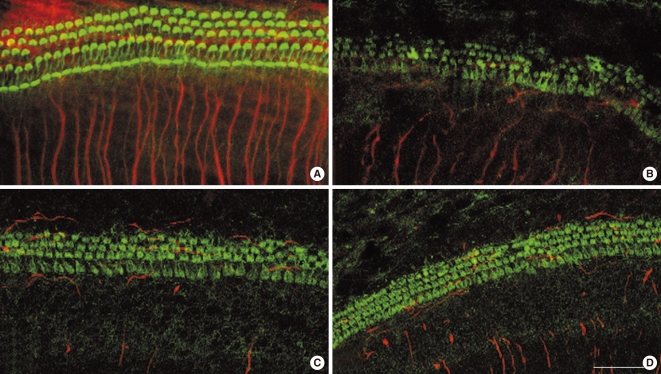
Fig. 2
Histogram shows mean number of outer hair cells (OHCs), per (A) and inner hair cells (IHCs) (B) per 0.2 mm length of the cochlea. Asterisk indicates that the carboplatin-treated group shows a statistically significant decrease in the OHC number compared to the control group. L-NAME: N-nitro-L-arginine methyl ester; L-NAC: N-acetyl-L-cysteine.
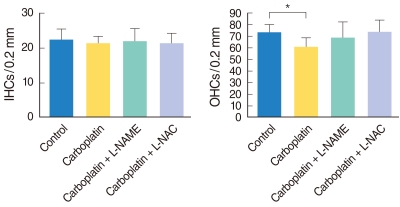
Fig. 3
Fluorescence microscopic photographs of dissociated spiral ganglion neuron cultures stained with NF-200 (green) and Hoechst (blue) after 72 hours of culture. (A) Untreated control, (B) 50 mg/mL carboplatin only, (C) 100 mM N-nitro-L-arginine methyl ester (L-NAME) only, (D) 100 mM N-acetyl-L-cysteine (L-NAC) only, (E) 50 mg/mL carboplatin+100 mM L-NAME, (F) 50 mg/mL carboplatin+100 mM L-NAC (×400).
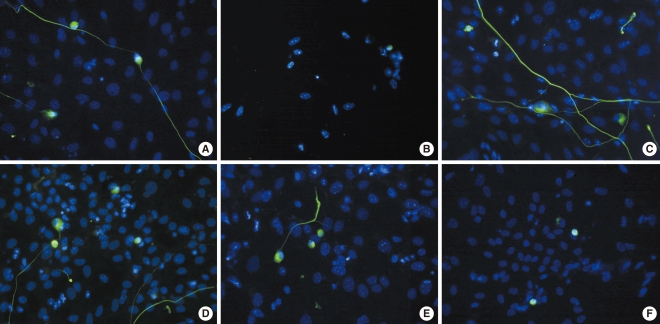
Fig. 4
Histogram shows relative percentages of NF-200 positive cells (survived spiral ganglion neurons) in the dissociated spiral ganglion neuron (SGN) cultures for 72 hours. The survival of SGNs in each condition is shown as a relative percentage of survival to control (100%). The Wilcoxon rank sum test was used to compare the surviving SGNs in the conditioned culture groups with the control group. L-NAME: N-nitro-L-arginine methyl ester; L-NAC: N-acetyl-L-cysteine.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download