Abstract
Objectives
Treating olfactory dysfunction is a challenge for physicians. One of the therapeutic options could be transplantation of stem cells. In this study, neural stem cells were transplanted into anosmic mice.
Methods
Neural stem cells were generated from the olfactory bulb of green fluorescent protein (GFP)-transgenic C57BL6 mice. Anosmia were induced by injection of intraperitoneal 3-methylindole. The neural stem cells were transplanted transnasally on the next day. The olfactory function was evaluated by a food-finding test once a week. The olfactory neuroepithelium was harvested for histologic examination and protein analysis at 4 weeks.
Results
Twenty-five percent (6/24) of the control mice that were not transplanted with neural stem cells survived at 4 weeks while 67% (8/12) of the transplanted mice survived (P=0.029). The food finding test showed that the transplanted mice resumed finding food at 3 weeks while the control mice resumed finding food at 4 weeks. GFP-positive cells were observed in the olfactory neuroepithelium of the transplanted mice. Western blotting revealed that the olfactory marker protein expression was significantly lower in the control mice than that in the transplanted mice.
Conclusion
This study demonstrated that improvement of mouse survival was achieved and recovery of olfactory function was promoted by transnasal transplantation of neural stem cells in the anosmic mouse model. These results indicate that stem cells might be one of the future modalities for treating olfactory impairment.
Olfactory impairment is one of the most common diseases of the sense organs and it is closely related to the quality of life, yet the importance of a sense of smell is frequently ignored during daily life (1, 2). The molecular mechanism of olfaction was quite recently brought to light and a Nobel Prize was awarded for this work (3). However, the pathogenic mechanism of olfactory impairment is still unclear, and so its treatment is challenging. Olfactory impairment can be caused by degeneration of olfactory receptor neurons in the nose and also by degeneration of the olfactory bulb or olfactory cortex (4).
The main etiologies of olfactory loss include sinonasal inflammation, viral infection of the upper respiratory tract and head trauma. All of the etiologies cause death of olfactory receptor neurons by different mechanisms. Throughout the whole life, olfactory receptor neurons are regularly regenerated from neural stem cells in the olfactory neuroepithelium (5). However, when regeneration fails, the subject will suffer from permanent olfactory impairment. The cells of the basal layer in the olfactory neuroepithelium are regarded as neural stem cells and their injury may result in olfactory dysfunction (6). Theoretically, restoration of the number of neural stem cells in the olfactory neuroepithelium or their function might be a good target for treating olfactory loss, but there are currently almost no drugs for this. Adult stem cells have been investigated as a new treatment method for various diseases such as myocardial infarction and stroke (7, 8). Cell-based therapy might be a promising option even for olfactory impairment. This study is aimed at investigating the efficacy of using neural stem cells for olfactory recovery in an anosmic mouse model.
Neural stem cells were prepared from the olfactory bulb of green fluorescent protein (GFP)-transgenic C57BL6 mice as previously described (9). All the experiments were performed with the approval of the Animal Care and Use Committee of Seoul National University Bundang Hospital. The olfactory bulbs were dissected out from the brain and mechanically dissociated by mincing. Enzymatic dissociation of the cells in the olfactory bulbs was performed by incubating the minced tissues in 0.25% trypsin (Sigma, St Louis, MO, USA) for 10 min at 37℃. Thereafter, the tissues were gently triturated and washed in Hank's balanced salt solution (Invitrogen, San Diego, CA, USA). The dissociated cells were filtered through 40 µm nylon mesh (Millipore, Billerica, MA, USA) to make a suspension of single cells. After washing, the pellet of single cells was resuspended in DMEM/F-12 culture medium (Invitrogen) supplemented with N-2 (R&D Systems, Minneapolis, MN, USA), 20 ng/mL epidermal growth factor (EGF, R&D Systems), 20 ng/mL basic fibroblast growth factor (bFGF, R&D Systems) and penicillin/streptomycin (Sigma). The cells were plated onto a 12-well plastic plate (Nunc, Rochester, NY, USA) and maintained at 37℃ in 5% CO2. The cells were immunostained with anti-nestin antibody (Chemicon, Temecula, CA, USA) to identify their characteristics as neural stem cells.
The olfactory ability of the mice was evaluated by a food-finding test. All the mice were acclimated to the animal laboratory environment for two weeks. During the acclimation period, the mice were regularly fed a piece of cheese once a day. Before the food-finding test, the mice were deprived of all the food, except water, for 72 hr and then one mouse was placed in a cage to find the piece of cheese buried beneath the wood shavings. The test was performed four times every 15 min. The mouse was left in the cage to find the cheese for up to 2 min. All the examined mice found the cheese within 15 sec before induction of anosmia.
Anosmia was induced in thirty-six 6-week-old female C57BL6 mice by intraperitoneal injection of 3-methylindole (6,000 µg/mouse). On the next day, 500,000 neural stem cells suspended in 50 µL of culture media were transnasally transplanted in twelve mice. Ten microliter micropipette tips were used for intranasal transplantation. Five microliters at a time were alternatively administered on each side to prevent asphyxia due to aspiration. The same amount of simple culture media was administered in the other twenty-four mice as controls. The olfactory ability test was performed every week and the mice were sacrificed at 4 weeks after injection of 3-methylindole. The olfactory neuroepithelium was obtained for histologic examination or immunoblotting.
To confirm the presence of transplanted neural stem cells in the olfactory neuroepithelium without any staining process, the whole olfactory neuroepithelium was microscopically dissected after bisection of the head and the olfactory neuroepithelium was elevated from the underlining ethmoturbinate of the transplanted mice 4 weeks after transplantation. The whole tissue was mounted on slides and the green fluorescence, which represents transplanted neural stem cells, was observed.
Immunohistochemistry and Western blot assay were performed as previously described. Briefly, for immunohistochemical staining, the heads of the mice, including the olfactory neuroepithelium, were fixed in 4% paraformaldehyde (Sigma) overnight. Thereafter, the head was decalcified with Decalcifier (National Diagnostics, Atlanta, GA, USA) at room temperature (RT) for 5 hr and then it was rinsed with distilled water for 15 min four times. Cryoprotection of the specimens was performed using sucrose in phosphate buffered saline (PBS). The specimens were serially placed in 10% sucrose for 1 hr, in 20% sucrose for 1 hr and finally in 30% sucrose overnight in a cold room. The specimens were embedded in ornithine carbamyl transferase compound and stored at -20℃. The frozen specimens were cut 5 µm in thickness and these sections were immediately mounted on slides. Immunohistochemistry was performed using an Ultravision Immunohistochemistry detection kit (Lab Vision, Fremont, CA, USA).
For staining the olfactory marker protein (OMP), the sections were incubated with goat anti-OMP (1:10,000; Wako Pure Chemicals, Osaka, Japan) at 4℃ overnight. Biotinylated rabbit anti-goat IgG (Zymed, San Francisco, CA, USA) was then applied and incubated at RT for 10 min. For visualization, the sections were incubated with horseradish peroxidase-conjugated streptavidin at RT for 10 min. For contrast, the sections were also stained with hematoxylin.
Since the green fluroscence was quenched with the staining process, the GFP was stained with an antibody against GFP. The staining for GFP was performed on a section just adjacent to the section stained for OMP. The section was incubated with rabbit anti-GFP (1:1,000; Novus Biologicals, Littleton, CO, USA) at 4℃ overnight, and biotinylated goat anti-rabbit IgG (Labvision) was then applied and incubated at RT for 10 min. The control staining was performed on sections from a GFP-transgenic mouse and a normal mouse.
The OMP levels were examined by immunoblotting 4 weeks after transplanting the neural stem cells. Olfactory neuroepithelium were collected for immunoblotting. After the mice were sacrificed, the ethmoturbinate was removed, the olfactory neuroepitheium was microdissected under a microsurgical microscope and then it was quickly placed in a liquid nitrogen tank. The microdissected olfactory neuroepithelium was homogenized and the protein was extracted using protein extraction buffer (Intron, Seoul, Korea).
The olfactory neuroepithelium samples were subjected to 15% SDS-PAGE and the proteins were transferred to a polyvinylidene fluoride membrane. The reliability of sample loading and electroblotting in each experiment was evaluated by staining nitrocellulose membranes with Ponceau S (Bio-Rad, Hercules, CA, USA) before immunoblotting. If the transfer was not uniform, then the blots were discarded and the gels were run again. The blots were blocked with 5% nonfat dry milk with 0.1% Tween 20 in 50 mM Tris-buffered saline (pH 7.4) and incubated overnight at 4℃ with a goat anti-OMP antibody. After the primary antibody incubation, the blots were washed and incubated with horseradish peroxidase-conjugated donkey anti-goat IgG (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). Thereafter, the blots were developed with enhanced chemiluminescence (Amersham, Arlington Heights, IL, USA) and exposed to x-ray film. The blots were then reprobed with an antibody to α-tubulin (Labfrontier, Seoul, Korea) as a control for protein loading. The signal intensity of Western blotting was quantified by densitometry using the Bio-image analyzer (Vilber Lournat, Cedex, France) and it was normalized to the corresponding signal for α-tubulin. The relative density of the OMP protein band compared to that of α-tubulin was calculated.
The Kaplan-Meier method was used to estimate the probability of survival, with death being the event of interest. The non-parametric log-rank test was employed to compare the survival curves. The time spent until the food was found, as well as the relative densities of OMP on the western blotting, was compared by student's t-test. P-values less than 0.05 for the statistical tests were considered statistically significant.
The cultured olfactory bulb-derived cells formed neurospheres and they were successfully were passaged. The cells showed green fluorescence since the cells originated from GFP-transgenic mice (Fig. 1A). Immunostaining of the neurospheres with nestin, which is a neural stem cell marker, showed that the neurosphere-forming cells were neural stem cells (Fig. 1B).
Twenty-five percent (6/24) of the control mice that were not transplanted with neural stem cells survived at 4 weeks after injection of 3-methylindole. On the other hand, it is quite interesting that 67% (8/12) of the transplanted mice survived at 4 weeks (P=0.029) (Fig. 2). During the 4 weeks, the transplanted mice have looked healthier than the control mice.
The olfactory function of the mice was evaluated by the food finding test. Five out of six transplant mice could find the food at 3 weeks while two out of six control mice could find the food at 3 weeks (Fig. 3). At 3 weeks, the time spent to find the food was 105±25 sec for the control mice and it was 74±34 sec in the transplanted mice (P=0.13). At 4 weeks, the time spent to find the food was 79±29 sec for the control mice whereas it was 42±20 sec for the transplanted mice (P=0.04).
The whole tissue mounting of the olfactory neuroepithelium on the slides demonstrated several conglomerates of green fluorescent neural stem cells that had been transplanted and they resided in the olfactory neuroepithelium (Fig. 4).
OMP-positive or GFP-positive cells were observed on the immunohistochemical staining of the consecutive sections of the olfactory turbinates. OMP-positive cells were observed in the olfactory neuroepithelium from the healthy wild-type mice that were not treated with 3-mehtylindole and that had normal olfactory function, but there were no GFP-positive cells (Fig. 5A, 5B). In the olfactory neuroepithelium from the anosmic mice that were not transplanted with neural stem cells, there were no OMP- or GFP-positive cells (Fig. 5C, 5D). In the transplanted mice, there were no GFP-positive cells in some of the olfactory neuroepithelial regions (Fig. 5E, 5F), while cells positive for both OMP and GFP were observed in some regions (Fig. 5G, 5H).
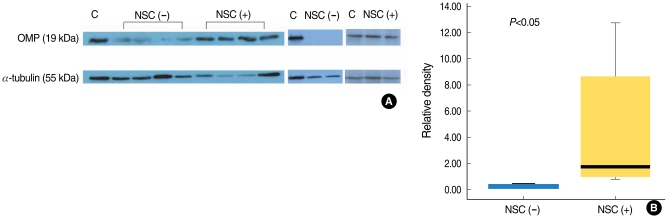
Western immunoblotting of the olfactory neuroepithelium showed a significant difference of the OMP expression between the control and transplanted mice (Fig. 6). The expression of OMP was significantly higher in the transplanted mice than that in the control mice.
Transplantation of stem cells has been suggested to be one of the innovative future treatment modalities for various untreatable diseases (10). One of the target diseases is neurodegenerative diseases such as Parkinson's disease (11-13) and Alzheimer's disease (14, 15). Anosmia is also one of the neurodegenerative diseases that cannot be treated by the current medical or surgical techniques. Olfactory receptor neurons are known to regenerate throughout the lifetime because there are some cells in the basal layer of the epithelium that play a role as neural stem cells. However, some patients suffer from permanent olfactory loss in spite of the regenerative potential of the olfactory neuroepithelium, and this may be explained when there is a significant reduction of the stem cell population in the epithelium.
There have been some previous trials to transplant stem cells in the olfactory neuroepithelium. Human cord blood CD133-positive stem cells were intravenously injected to nod-scid mice and this was shown to provide conditions for regeneration of the olfactory neuroepithelium after permanent damage was induced by dichlobenil (16). Adipose tissue-derived stem cells were also intravenously injected to rats after their olfactory nerve was transected (17). However, the olfactory nerve transaction model is less likely to be appropriate for stem cell research. It is because when an efferent neural signal pathway from the upper level of the olfactory nervous system is interrupted, it finally leads to death of the lower level of cells and specifically the olfactory neuronal cells that have differentiated from the injected stem cells. A study using bone marrow cells of GFP mice revealed that bone marrow cells can be engrafted in the olfactory epithelium of the irradiated mice and then be differentiated into olfactory neuronal cells (18).
As shown in the above-mentioned studies, most of the stem cell transplantation trials were performed via a parenteral route using mesenchymal tissue-derived stem cells. However, systemic administration of stem cells has disadvantages. A large part of the cells will be trapped in the lung and therefore cannot reach the target tissue (olfactory neuroepithelium) (19). This means that a large number of cells are required to achieve therapeutic effects. Neural stem cells might have more benefits than mesenchymal tissue-derived stem cells in that neural stem cells might be more easily differentiated into olfactory receptor neurons because both of them are from the same neural lineage. In order to overcome those disadvantages, neural stem cells were used and administered directly into the nasal cavity in our study. Generally, stem cells are known to 'home' to the site of injury (20). The olfactory neuroepithelium can be completely de-epithelialized by intravenous injection of 3-mehtylindole. Therefore, the injected neural stem cells might directly reside in the olfactory neuroepithelium without a massive loss of the cells in the lung.
Out study showed a very interesting finding in terms of the survival of the mice. Transplantation of neural stem cells was associated with prolongation of survival in the anosmic mice. The transplanted mice survived significantly longer than the nontransplanted mice. Because the sense of smell is much more important for survival in mice than in humans, the anosmic mice may be more subject to death than the mice with normal olfactory function. Better survival in the transplanted mice might suggest that they have better recovery from the anosmic condition by transplantation of neural stem cells than the nontransplanted mice. These results were also supported by other data in our study. Transplantation of neural stem cells was associated with better recovery of olfactory function in terms of the food-finding test and the expression of OMP. However, this study has a weakness that the anosmic mice had acute olfactory injury instead of chronic injury, and the latter is more common in the actual medical situation. Therefore, further studies are required to identify the therapeutic role of stem cells in long-lasting olfactory injury.
This study demonstrated that improvement of mouse survival was achieved and recovery of olfactory function was promoted by transnasal transplantation of neural stem cells in the anosmic mouse model. These results indicate that stem cells might be one of the modalities for treating olfactory impairment in the future.
ACKNOWLEDGMENTS
This study was partly supported by the Korean Research Foundation Grant funded by the Korean Government (MOEHRD) (grant # KRF-2007-313-E00316) and a SNUBH grant # 02-2007-035.
References
1. Lin SH, Chu ST, Yuan BC, Shu CH. Survey of the frequency of olfactory dysfunction in Taiwan. J Chin Med Assoc. 2009; 2. 72(2):68–71. PMID: 19251533.

2. Doty RL. The olfactory system and its disorders. Semin Neurol. 2009; 2. 29(1):74–81. PMID: 19214935.

3. Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991; 4. 65(1):175–187. PMID: 1840504.

4. Nordin S, Bramerson A. Complaints of olfactory disorders: epidemiology, assessment and clinical implications. Curr Opin Allergy Clin Immunol. 2008; 2. 8(1):10–15. PMID: 18188011.

5. Beites CL, Kawauchi S, Crocker CE, Calof AL. Identification and molecular regulation of neural stem cells in the olfactory epithelium. Exp Cell Res. 2005; 6. 306(2):309–316. PMID: 15925585.

6. Iwai N, Zhou Z, Roop DR, Behringer RR. Horizontal basal cells are multipotent progenitors in normal and injured adult olfactory epithelium. Stem Cells. 2008; 5. 26(5):1298–1306. PMID: 18308944.

7. Dimmeler S, Zeiher AM. Cell therapy of acute myocardial infarction: open questions. Cardiology. 2009; 113(3):155–160. PMID: 19122455.

8. Andres RH, Choi R, Steinberg GK, Guzman R. Potential of adult neural stem cells in stroke therapy. Regen Med. 2008; 11. 3(6):893–905. PMID: 18947311.
9. Ahn JM, Lee CH, Kim DY, Rhee CS, Min YG, Kim JW. Maintenance of regional difference in cellular composition of neurospheres derived from adult mouse olfactory bulb. Eur Arch Otorhinolaryngol. 2008; 4. 265(4):429–434. PMID: 17938947.

10. Kim SU, de Vellis J. Stem cell-based cell therapy in neurological diseases: a review. J Neurosci Res. 2009; 8. 87(10):2183–2200. PMID: 19301431.

11. Madhavan L, Daley BF, Paumier KL, Collier TJ. Transplantation of subventricular zone neural precursors induces an endogenous precursor cell response in a rat model of Parkinson's disease. J Comp Neurol. 2009; 7. 515(1):102–115. PMID: 19399899.

12. Greene P. Cell-based therapies in Parkinson's disease. Curr Neurol Neurosci Rep. 2009; 7. 9(4):292–297. PMID: 19515281.

13. Lindvall O, Kokaia Z. Prospects of stem cell therapy for replacing dopamine neurons in Parkinson's disease. Trends Pharmacol Sci. 2009; 5. 30(5):260–267. PMID: 19362379.

14. Xuan AG, Luo M, Ji WD, Jin WD, Long DH. Effects of engrafted neural stem cells in Alzheimer's disease rats. Neurosci Lett. 2009; 1. 450(2):167–171. PMID: 19070649.

15. Mimeault M, Batra SK. Recent progress on tissue-resident adult stem cell biology and their therapeutic implications. Stem Cell Rev. 2008; Spring. 4(1):27–49. PMID: 18288619.

16. Franceschini V, Bettini S, Pifferi S, Rosellini A, Menini A, Saccardi R, et al. Human cord blood CD133+ stem cells transplanted to nodscid mice provide conditions for regeneration of olfactory neuroepithelium after permanent damage induced by dichlobenil. Stem Cells. 2009; 4. 27(4):825–835. PMID: 19350683.

17. Kim YM, Choi YS, Choi JW, Park YH, Koo BS, Roh HJ, et al. Effects of systemic transplantation of adipose tissue-derived stem cells on olfactory epithelium regeneration. Laryngoscope. 2009; 5. 119(5):993–999. PMID: 19296495.

18. Tsujigiwa H, Nishizaki K, Teshima T, Takeda Y, Yoshinobu J, Takeuchi A, et al. The engraftment of transplanted bone marrow-derived cells into the olfactory epithelium. Brain Res. 2005; 8. 1052(1):10–15. PMID: 15996641.

19. Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009; 6. 18(5):683–692. PMID: 19099374.

20. Smart N, Riley PR. The stem cell movement. Circ Res. 2008; 5. 102(10):1155–1168. PMID: 18497316.

Fig. 1
Neurospheres from the olfactory bulb of the green fluorescence protein-transgenic C57BL6 mice (A). The neural stem cells have formed neurospheres floating in the culture media. Immunoreactivity to a marker for neural stem cells (B). The neurosphere-forming cells were immunoreactive to nestin (red), which is a neural stem cell marker.
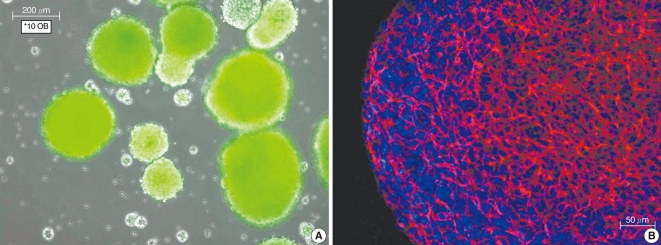
Fig. 2
The survival analysis. Six out of twenty-four control mice that were not transplanted with neural stem cells (NSC) survived at 4 weeks after injection of 3-methylindole, while 8 out of the 12 transplanted mice survived at 4 weeks (P=0.029).
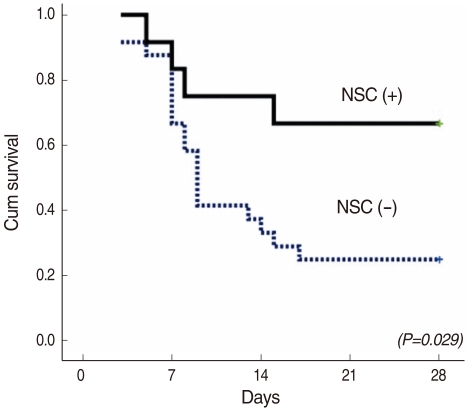
Fig. 3
The olfactory function. The olfactory function was evaluated by the time spent to find a piece of cheese buried beneath the wood shavings. Five out of six transplanted mice (right) with neural stem cells (NSC) could find the food at 3 weeks while two out of six control mice could find the food at 3 weeks (left). At 3 weeks, there was no significant difference in the time spent to find the cheese between the control and transplanted mice (P=0.13). On the other hand, at 4 weeks, the cheese was found more quickly by the transplanted mice than by the control mice (P=0.04). NSC (-) denotes without transplantation of NSC while NSC (+) with transplantation.
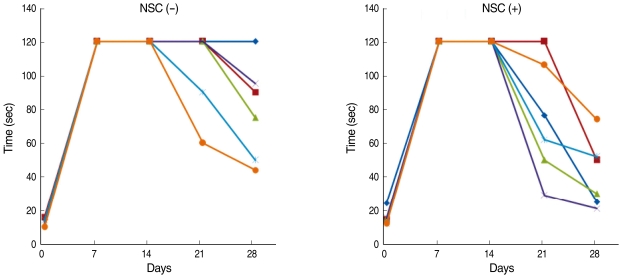
Fig. 4
Neural stem cells in the whole-mount olfactory neuroepithelium. The whole tissue mounting showed several conglomerates of green fluorescent neural stem cells that had been transplanted and they resided in the olfactory neuroepithlium.
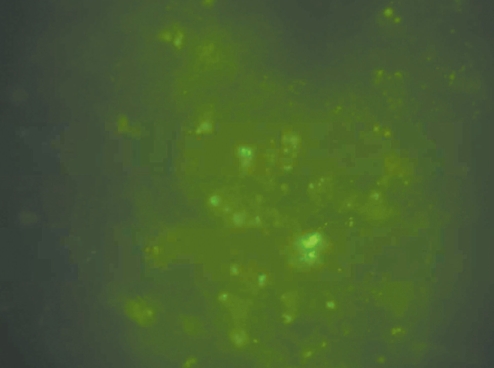
Fig. 5
Immunohistochemical staining for olfactory marker protein (OMP) and green fluorescent protein (GFP). The staining was performed on the consecutive sections of the olfactory turbinates. OMP-positive cells were observed (A) in the olfactory neuroepithelium from the healthy wild-type mice that were not treated with 3-mehtylindole and that had normal olfactory function, while there were no GFP-positive cells (B). There were no OMP- (C) or GFP-positive cells (D) in the olfactory neuroepithelium from the anosmic mice that were not transplanted with neural stem cells. In the transplanted mice, the cells were only positive for OMP (E) but not for GFP (F) in some olfactory neuroepithelial regions, whereas cells that were positive for both OMP (G) and GFP (H) were observed in some regions.
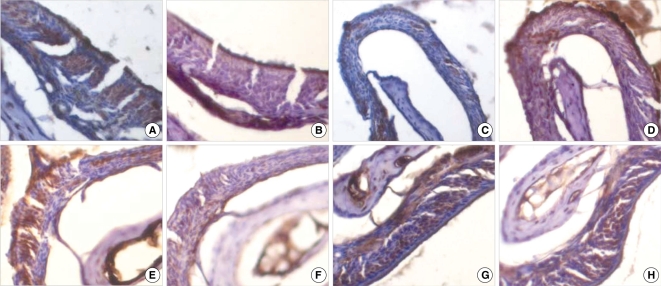
Fig. 6
Western immunoblotting of the olfactory neuroepithelium. Olfactory marker protein (OMP) was more highly expressed in the transplanted mice than that in the control mice (A). Densitometric analysis showed a significant difference of the OMP expression between the control and transplanted mice (B). neural stem cells (NSC) (-) denotes without transplantation of neural stem cells while NSC (+) with transplantation.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download