Abstract
Objectives
The objective of this study was to determine the various factors that affect the extrusion rate of ventilation tubes (VTs), including the nature of the middle ear effusion.
Methods
A retrospective chart review of 82 pediatric patients (177 ears) who received VT insertion surgery under general anesthesia was carried out to evaluate the relationship between various factors and the VT extrusion rate. The factors we analyzed included age, gender, the adenoid size, the amount and content of the middle ear effusion after myringotomy, bleeding events, associated adenoidectomy and the findings of the tympanic membrane status, the tympanometry and the audiometry of the air bone gap.
Results
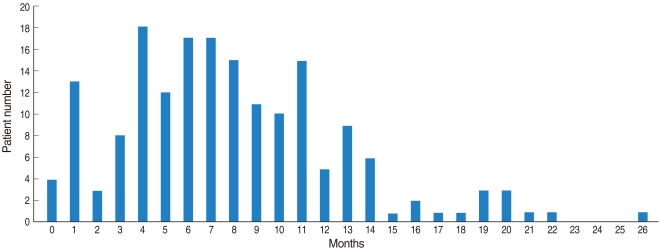
The mean extrusion time was 254 days (range, 11 to 809 days). The patients with no history of previous VT insertion had a longer extrusion time (mean, 279 days) than did the patients who had undergone previous VT insertion (mean, 203 days). The patients with serous effusion had the shortest extrusion time (mean, 190 days) as compared to those patients with glue (273 days) and pus (295 days) effusions. Other factors had no statistical significant relationship with the extrusion time.
Go to : 
Otitis media with effusion (OME) is common in the pediatric population. More than 80% of all children will have experienced OME by the age of 4. In many published studies, the peak incidence of otitis appears to be in the 6- to 36-month age group, whereas the median age for placement of the ventilation tube (VT) is about 18 months (1). For recurrent OME, it may be necessary to insert a VT that provides long-term ventilation of the middle ear to prevent the development of tympanic membrane retraction, ossicular erosion or formation of a retraction pocket.
Many factors have been suggested that may affect the VT function and extrusion. The reported factors include a previous history of VT insertion (1) the status of the tympanic membrane (2), the location of the myringotomy incision (3), the side of the VT insertion and a repeated episode of otorrhea (4), looseness of the VT at the time of insertion and the shape of the VT (5), and there are some discrepancies among the reports. There is limited information on the relationship between the characteristics of middle ear effusion and the VT extrusion time. The objective of this study was to determine the various factors that affect the VT extrusion rate, including the nature of the middle ear effusion.
Go to : 
A retrospective chart review was carried out for the pediatric patients who received VT insertion surgery at our department from July 2001 to April 2008. We excluded those adult cases, those with use of local anesthesia, those with infection of the VT and those with combined craniofacial anomaly. The medical charts of 82 patients (177 times of insertions) who had undergone their operation under general anesthesia and who could be followed up until the VT had extruded after surgery were retrospectively reviewed. For all the patients, a myringotomy with insertion of a VT (Paparella type I, Medtronic Xomed, Jacksonville, FL, USA) was performed under general anesthesia. The patients were evaluated one week and one month after the operation, and thereafter every three months at the outpatient clinic.
The date of extrusion was calculated as the midpoint between the last date when the VT was seen to be in situ and the first date when the VT was seen to be extruded. We evaluated the age, gender, associated adenoidectomy, the adenoid size, the amount and content of the middle ear effusion after myringotomy, bleeding events and the findings of the tympanic membrane status, the tympanometry and the audiometry of the air bone gap. The patients were divided into two groups based on the age of 7. An independent t-test was used to compare the extrusion time between the patients who were younger (<7 yr) and those who were older (≥7 yr), the males and females, those with/without a previous history of VT insertion and those who had/had not undergone adenoidectomy. Before the operation, tympanometry was performed and the audiometry was evaluated. The threshold of hearing was calculated by the 4 division method, and then the air bone gap (dB) was calculated. The VT extrusion time was compared for the patients with preoperative tympanometry B and C and whose air bone gap on audiometry before the operation was 20 dB or smaller vs. those patients with preoperative tympanometry B and C and whose air bone gap on audiometry before the operation larger than 20 dB.
The adenoid size was measured for each patient before the operation. The lateral skull radiography was taken, and adenoid hypertrophy was measured using the adenoidal nasopharyngeal ratio (6). The measured ratio was categorized in this way: under 0.25 as grade 0, 0.26 to 0.5 as grade 1, 0.51 to 0.75 as grade 2 and over 0.75 as grade 3. The tympanic membrane (TM) was evaluated before the operation using a 0° endoscope. The TM was classified as retraction, bulging, hyperemia and effusion. During the operation, the characteristics of middle ear effusion were recorded and classified as serous, mucoid, puslike or glue. The characteristics of the effusion were determined by the operator. The amount of effusion after myringotomy during VT insertion was measured and this was recorded as absent or scanty vs. profuse. The extrusion time was compared between the groups by using one-way analysis of variance (ANOVA) according to the adenoid size, the TM status, the characteristics of middle ear effusion and the amount of effusion. Data analysis and statistical tests were performed using the SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). An independent t-test, ANOVA, multivariate analysis and multiple regression analysis were used for statistical analysis. Statistical significance was accepted at the level of P<0.05. The statistical analyses were performed by an independent statistician in the Medical Research Collaborating Center.
Go to : 
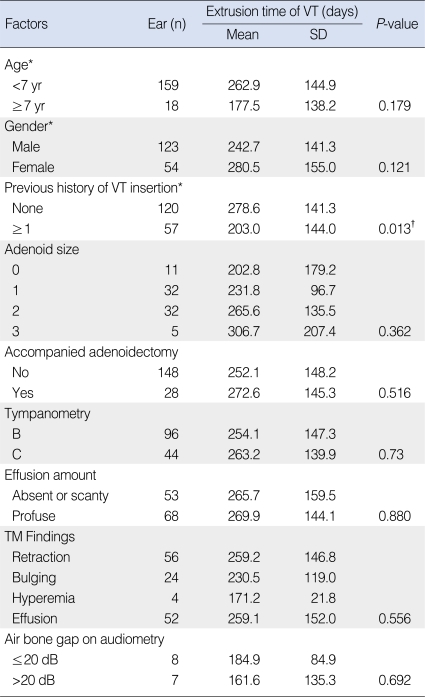
The total number of VT insertions was 177 and this number included 30 ears with multiple insertions. The median age of the patients at the time of VT insertion was 4.72 yr (range, 1.2 to 11.0 yr). The mean extrusion time was 254 days (range, 11 to 809 days) (Fig. 1). The statistical analysis for the various factors and extrusion times is shown in Table 1. When a P-value of 0.2 was set as the criterion, age (< 7 yr vs. ≥7 yr), gender (male vs. female), a previous history of VT insertion (none vs. more than once) and the effusion characteristics (serous, mucoid, pus, glue) were chosen as factors to evaluate on multivariate analysis. Multiple regression analysis was carried out since there was no relationship between the factors according to multicollinearity. The change of the extrusion time was statistically significantly explained by a suitable regression line (P=0.0001).
The extrusion time of the VT had a tendency to be longer for the younger patients (age, <7 yr old; mean extrusion time, 263 days) than that for the older patients (age, ≥7 yr old; mean extrusion time, 177 days) on univariate analysis, but this was not significantly different (P=0.180, multiple regression). The extrusion time was not significantly different between the males and females (243 days vs. 281 days, respectively; P=0.121, multiple regression analysis).
The patients with no history of VT insertion had a longer extrusion time than that of the patients who had undergone previous VT insertion (mean extrusion time, 279 days vs. 203 days, respectively), and this difference was statistically significant (P=0.013, multiple regression analysis). There was a decrease of the extrusion time of 59.62 days for the patients with a previous history of VT insertion as compared with that of the patients who underwent VT insertion for the first time.
The extrusion time tended to increase with an increase of adenoid hypertrophy (grade 0, 202 days; grade 1, 231 days; grade 2, 266 days; grade 3, 307 days). However, there was no statistical significance (P=0.362). There was no difference in the extrusion time between the patients who had VT insertion accompanied by adenoidectomy (mean, 272 days) and those who had VT insertion without adenoidectomy (mean, 252 days; P=0.516).
No significant difference of the extrusion time was found among the four groups based on the TM status: retraction (259 days), bulging (231 days), hyperemia (171 days) and effusion (259 days) (P=0.556). No difference of the extrusion time was found between the class B impedance group (254 days) and the class C impedance group (263 days, P=0.73). There was no difference between the two air bone gap groups: AB gap ≤20 dB (185 days) and AB gap>20 dB (162 days) (P=0.692).
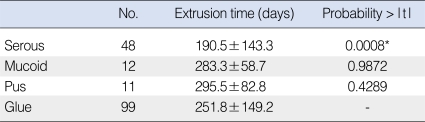
The patients with serous effusion had the shortest extrusion time (mean, 190 days), compared to those with glue-like (273 days), mucoid (283 days) and pus-like (295 days) effusions (Table 2). When we set the dummy variables to the glue-like group, there was a significant difference between the patients with serous and glue-like (P<0.001) effusions, and there was no differences between the patients with glue-like and pus (P=0.429) effusions, and those with glue-like and mucoid effusion (P=0.987). The serous and pus groups also showed a significant difference for the extrusion time (P=0.124). The mucoid group did not show a significant difference from the serous group for the extrusion time (P=0.0675). This showed that the serous group was different from the other groups on multiple regression analysis. There was no significant relationship between the extrusion time and the amount of effusion (265 days [absent or scanty] vs. 267 days [profuse], P=0.880).
Go to : 
There have been several studies that have evaluated the factors affecting the VT extrusion time, but there is no consensus and the results have been diverse. While our primary goal was to determine the effect of the characteristics of middle ear effusion on the VT extrusion time, this study provided an excellent opportunity to monitor other parameters that might also influence the extrusion time.
Gibb and Mackenzie (7) reported that age had no significant effect on extrusion. In contrast, Leopold and McCabe (2) reported a negative correlation between the longevity of tube functioning and an age ≥10 yr. Various factors associated with age have been found to be correlated with OME. The prevalence of OME and Eustachian tube (ET) growth are related to age. The incidence of OME recurrence decreases when the patient's age at VT removal or extrusion is 7-8 yr (8). Bylander et al. (9) and Tos et al. (10) reported an improvement of the ET function as well as a drastic fall in the prevalence of secretory otitis to 7% at the age of 6-7. We expected that the extrusion time of VT may be related to the link between age and the growth and function of the ET, but our study showed no difference between patients under and over the age of 7.
Leopold and McCabe (2) reported that the VT remained in the TM of the patients with no history of previous VT insertion significantly longer than that in the patients who were previously intubated. Our study had the same findings, that is, there was a longer extrusion time for the VT insertion patients with no previous history of VT insertion (279 days) than that of the patients who had undergone previous intubation (203 days).
The types of middle ear effusion vary from serous fluid to thick glue-like secretions. Carrie et al. reported that the viscosity of mucoid effusions was significantly greater than the viscosity of serous effusions, and the only constituent in effusions that determines the viscosity is mucin, not protein or lipid. They concluded that thick (mucoid) and thin (serous) middle ear effusions can be classified on the basis of visible inspection and the flow properties (11).
Allen et al. (12) reported that the children at high risk for developing postoperative complications after VT insertion were more likely to have mucopus in the ear at the time of surgery. Ahn et al. (13) reported that the percentage of glue-like effusion was decreased with repeated VT insertion procedures. A previous study suggested that different effusions with various levels of enzymes and proteins were correlated with recurrent cases of OME (14). In contrast, Gibb and Mackenzie (7) and Maw et al. (15) reported that there was no significant difference of the VT extrusion rate when the middle ear fluid was divided into liquid, glue and very thick glue.
The role of cytokines, which is a group of glycoproteins that participates in the modulation of inflammatory and immune reactions in many diseases, has recently been emphasized in OME (16). Earlier studies have documented the presence of several cytokines in the middle ear effusions of humans and experimental animals, including tumor necrosis factor (TNF)α, interleukin (IL) 1β, IL-2, IL-6, and IL-8, interferon (IFN)-γ and TNF soluble receptor (17-19). Kariya et al. (20) compared the cytokine expression according to the type of effusion. The concentration and incidence rate of IL-10 in mucoid OME were significantly higher than those in serous OME. However, other cytokines (IL-2, IL-4, IL-5, IL-12, and IFN-γ) were not significantly different between the two groups. Hotomi et al. (18) also reported that the mean level of IL-8 in serous effusion was lower than that in the other two types of MEE (mucoserous and mucoid) (18). TNF-α may be associated with histopathological changes in the middle ear mucosa and the production of mucoid OME in patients with persistent chronic OME. Maeda et al. inoculated TNF-α and endotoxin into the middle ear cavity of rats, followed by ET obstruction, and this resulted in mucous cell metaplasia-hyperplasia that was accompanied by abundant mucin or mucin-like glycoproteins in middle ear effusions (21). Similarly, Smimova et al. (22) reported that the mucin content of effusions was correlated with the concentration of IL-4 and IL-13, suggesting the involvement of IL-4 and IL-13 in the upregulation of middle ear mucin metabolism. The IL-8 concentration was positively correlated with the type of effusion and the total number of neutrophils in OME (18).
In our study, the extrusion time in the serous effusion group (191 days) was shorter than that in the mucoid (283 days), pus (296 days) and glue (252 days) effusion groups. Serous effusion has a lower viscosity as compared to that of glue, pus and mucoid effusions. Takahashi et al. (23) suggested that viscous effusion aggravates the ET function as a result of OME. In evaluating the pathogenesis of the course of OME, Hormann concluded that the viscosity of the effusion reflects the stage and progression of OME (24). A serous effusion possibly suggests an early stage of OME and the patients with a serous effusion have better ET function; the patients with serous OME heal faster than do the patients with OME with highly viscous effusion (which is in a more advanced stage of the disease with a higher mucin concentration and cytokine interaction).
The amount of effusion during VT insertion (absent or scanty vs. profuse) had no significant effect on the extrusion time (P=0.880). Jeon et al. (25) reported that blockage of VT in the immediate postoperative period had no significant effect on the amount of middle ear effusion that was graded as dry, small, moderate and large.
Adenoid hypertrophy and chronic or recurrent adenoiditis have been postulated as contributing factors in the development of recurrent AOM and chronic OME (26). Yet in relation to the removal of the adenoid, Iwaki et al. (8) reported that adenoidectomy did not influence the recurrence rate of OME. Similarly, Mackenzie (5) and Gibb and Mackenzie (7) reported that simultaneous operative procedures, including adenoidectomy, had no significant effect on the extrusion rate of VT. Our study had corresponding results with no difference of the extrusion time between the patients who had an accompanying adenoidectomy (252 days) and those who did not (272 days, P=0.516), and there was no difference according to the adenoid size.
Sichel et al. (27) analyzed the viscosity of ear effusion and tympanometry, along with the AB gap on audiometry. No correlation was found between the viscosity of the middle ear fluid and the three parameters of tympanometry as well as between the viscosity and the AB gap. In our study, there was no significant relationship between the preoperative tympanometry B and C groups (P=0.73). Also, no difference was found between the patients in the AB gap 20 dB or smaller group and the group of patients with an AB gap larger than 20 dB (P=0.692).
Mackenzie (5) reported that the quality of the TM did not affect the extrusion rate. Gibb and Mackenzie (7) analyzed the TM as normal, thin scarred, tympanosclerosis, thick and gross retraction; they concluded that the TM status did not affect the extrusion rate. In our study, the TMs were grouped as retraction, bulging, hyperemia and effusion, and there was no significant relationship (P=0.556).
In our study, gender was not significantly related to the extrusion time (P=0.114), which corresponds to the previous reports (5, 7). Bleeding during VT insertion did not have a significant relationship with the extrusion time (P=0.143), which is also consistent with previous reports (5, 7, 25).
The design of the VT has been reported to be a significant determinant of the extrusion rate without any debate (2, 7, 28, 29). Our study was confined to the Paparella type I VT (Medtronic Xomed, Jacksonville, FL, USA). Operator experience and general anesthesia are factors that have been reported to prolong the VT extrusion time (2, 5). There is also controversy about the effect of the quadrant of the TM where the VT is inserted on the extrusion time. Shah (3) reported that inserting the VT in the anterior segment of the TM prolongs the VT functioning time. However, Mackenzie (5) and Hern and Jonathan (28) reported that the portion of the TM portion where the TV is incised had no significant effect. Otic drop use, left ear surgery, repeated otorrhea episode, a loose VT at insertion and the presence of concurrent infection have been reported to shorten the extrusion time (4, 5, 24, 25, 28).
The mean extrusion time of the VT was 254 days in our study. Two factors remained significant predictors of the extrusion time: the characteristics of the middle ear effusion and a history of previous VT insertion. No other factor appeared to influence the extrusion time. Additional prospective randomized studies with a large number of patients are needed to validate this finding. The nature of middle ear effusion can provide a clinical clue to predict the extrusion time of VT. More caution must be given to older patients and those with a previous history of VT insertion.
Go to : 
References
1. Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven yr of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989; 7. 160(1):83–94. PMID: 2732519.
2. Leopold DA, McCabe BF. Factors influencing tympanostomy tube function and extrusion: a study of 1,127 ears. Otolaryngol Head Neck Surg. 1980; Jul–Aug. 88(4):447–454. PMID: 6821430.
4. Valtonen H, Qvarnberg Y, Nuutinen J. Tympanostomy in young children with recurrent otitis media: a long-term follow-up study. J Laryngol Otol. 1999; 3. 113(3):207–211. PMID: 10435125.

5. Mackenzie IJ. Factors affecting the extrusion rates of ventilation tubes. J R Soc Med. 1984; 9. 77(9):751–753. PMID: 6541254.

6. Fujioka M, Young LW, Girdany BR. Radiographic evaluation of adenoidal size in children: adenoidal-nasopharyngeal ratio. AJR Am J Roentgenol. 1979; 9. 133(3):401–404. PMID: 111497.

7. Gibb AG, Mackenzie IJ. The extrusion rate of grommets. Otolaryngol Head Neck Surg. 1985; 12. 93(6):695–699. PMID: 3937089.

8. Iwaki E, Saito T, Tsuda G, Sugimoto C, Kimura Y, Takahashi N, et al. Timing for removal of tympanic ventilation tube in children. Auris Nasus Larynx. 1998; 12. 25(4):361–368. PMID: 9853658.

9. Bylander A, Ivarsson A, Tjernstrom O. Eustachian tube function in normal children and adults. Acta Otolaryngol. 1981; Nov–Dec. 92(5-6):481–491. PMID: 7315267.

10. Tos M, Holm-Jensen S, Stangerup SE, Sorensen CH. Changes in point prevalence of secretory otitis in preschool children. ORL J Otorhinolaryngol Relat Spec. 1983; 45(4):226–234. PMID: 6683833.

11. Carrie S, Hutton DA, Birchall JP, Green GG, Pearson JP. Otitis media with effusion: components which contribute to the viscous properties. Acta Otolaryngol. 1992; 112(3):504–511. PMID: 1441992.

12. Allen J, Morton RP, Ahmad Z. Early post-operative morbidity after tympanostomy tube insertion. J Laryngol Otol. 2005; 9. 119(9):699–703. PMID: 16156910.

13. Ahn JH, Yoon TH, Pae KH, Kim TS, Chung JW, Lee KS. Clinical manifestations and risk factors of children receiving triple ventilating tube insertions for treatment of recurrent otitis media with effusion. Pediatrics. 2006; 6. 117(6):e1119–e1123. PMID: 16702251.

14. Juhn SK. Studies on middle ear effusions. Laryngoscope. 1982; 3. 92(3):287–291. PMID: 7200175.
15. Maw AR, Bawden R, O'Keefe L, Gurr P. Does the type of middle ear aspirate have any prognostic significance in otitis media with effusion in children? Clin Otolaryngol Allied Sci. 1993; 10. 18(5):396–399. PMID: 8877207.

16. Skotnicka B, Hassmann E. Cytokines in children with otitis media with effusion. Eur Arch Otorhinolaryngol. 2000; 257(6):323–326. PMID: 10993552.

17. DeMaria TF, Murwin DM. Tumor necrosis factor during experimental lipopolysaccharide-induced otitis media. Laryngoscope. 1997; 3. 107(3):369–372. PMID: 9121315.

18. Hotomi M, Samukawa T, Yamanaka N. Interleukin-8 in otitis media with effusion. Acta Otolaryngol. 1994; 7. 114(4):406–409. PMID: 7976312.

19. Maxwell K, Leonard G, Kreutzer DL. Cytokine expression in otitis media with effusion: tumor necrosis factor soluble receptor. Arch Otolaryngol Head Neck Surg. 1997; 9. 123(9):984–988. PMID: 9305251.

20. Kariya S, Okano M, Hattori H, Sugata Y, Matsumoto R, Fukushima K, et al. TH1/TH2 and regulatory cytokines in adults with otitis media with effusion. Otol Neurotol. 2006; 12. 27(8):1089–1093. PMID: 16988618.

21. Maeda K, Hirano T, Ichimiya I, Kurono Y, Suzuki M, Mogi G. Cytokine expression in experimental chronic otitis media with effusion in mice. Laryngoscope. 2004; 11. 114(11):1967–1972. PMID: 15510024.

22. Smirnova MG, Birchall JP, Pearson JP. Evidence of T-helper cell 2 cytokine regulation of chronic otitis media with effusion. Acta Otolaryngol. 2005; 10. 125(10):1043–1050. PMID: 16298784.

23. Takahashi H, Honjo I, Yagi N, Kurata K. Viscosity of effusion in the middle ear and eustachian tube in patients with otitis media with effusion. Auris Nasus Larynx. 1990; 17(1):11–16. PMID: 2390028.

24. Hormann K. Pathogenesis and pathophysiology of middle ear effusions. Acta Otolaryngol Suppl. 1987; 440:1–59. PMID: 3480679.
25. Jeon EJ, Park YS, Lee SK, Chang KH, Park SY, Park KH, et al. Factors of the blockage of ventilation tubes in the immediate postoperative period. Eur Arch Otorhinolaryngol. 2007; 12. 264(12):1393–1397. PMID: 17657506.

26. Paradise JL, Bluestone CD, Rogers KD, Taylor FH, Colborn DK, Bachman RZ, et al. Efficacy of adenoidectomy for recurrent otitis media in children previously treated with tympanostomy-tube placement: results of parallel randomized and nonrandomized trials. JAMA. 1990; 4. 18. 263(15):2066–2073. PMID: 2181158.

27. Sichel JY, Priner Y, Weiss S, Levi H, Barshtein G, Eliashar R, et al. Characteristics of the type B tympanogram can predict the magnitude of the air-bone gap in otitis media with effusion. Ann Otol Rhinol Laryngol. 2003; 5. 112(5):450–454. PMID: 12784986.

28. Hern JD, Jonathan DA. Insertion of ventilation tubes: does the site matter? Clin Otolaryngol Allied Sci. 1999; 9. 24(5):424–425. PMID: 10542923.

29. Hussain SS. Extrusion rate of Shah and Shepard ventilation tubes in children. Ear Nose Throat J. 1992; 6. 71(6):273–275. PMID: 1451676.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download