Abstract
Objectives
The aim of this study was to investigate optimal continuous positive airway pressure (CPAP) level, to examine the factors affecting optimal CPAP level, and to develop a predictive equation for optimal CPAP level in Korean patients with obstructive sleep apnea syndrome (OSAS).
Methods
A total of 202 patients with OSAS who underwent successful manual titration for CPAP treatment were included in this study. Correlations between the optimal CPAP level and baseline data including anthropometric and polysomnographic variables were analyzed. A predictive equation for optimal CPAP level was developed based on anthropometric and polysomonographic data.
Results
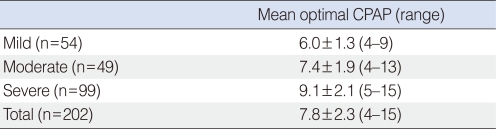
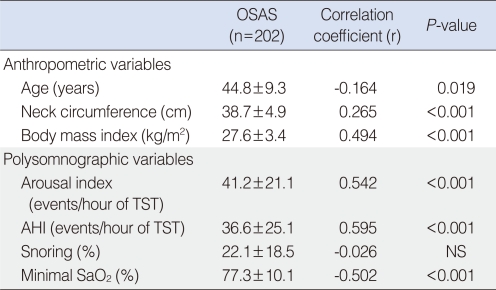
The mean optimal CPAP level in 202 patients with OSAS was 7.8±2.3 cm H2O. The mean optimal CPAP level in the mild, moderate, and severe OSAS groups was 6.0±1.3, 7.4±1.9, and 9.1±2.1 cm H2O, respectively. The apneahypopnea index (AHI) (r=0.595, P<0.001), arousal index (r=0.542, P<0.001), minimal SaO2 (r=-0.502, P<0.001), body mass index (BMI) (r=0.494, P<0.001), neck circumference (r=0.265, P<0.001), and age (r=-0.164, P=0.019) were significantly correlated with optimal CPAP level. The best predictive equation according to stepwise multiple linear regression analysis was: Optimal CPAP level (cm H2O)=0.681+(0.205×BMI)+(0.040×AHI). Forty-two percent of the variance in the optimal CPAP level was explained by this equation (R2=0.42, P<0.001).
Go to : 
Obstructive sleep apnea syndrome (OSAS) is a common chronic disease that requires long-term treatment and follow-up (1). The estimated prevalence of OSAS in middle-aged Koreans is 4.5% in males and 3.2% in females (2).
Treatment for OSAS consists of medical, behavioral and surgical therapy (1, 3). Medical therapy includes continuous positive airway pressure (CPAP) and oral appliances, and behavioral therapy consists of weight loss and positional therapy (1, 4-8). In 1981, Sullivan et al. (4) first introduced nasal CPAP, working as a pneumatic splint to prevent upper airway collapse, for OSAS treatment. CPAP is routinely recommended for treatment in patients with moderate to severe OSAS and optionally indicated for the treatment of patients with mild OSAS (9). After CPAP treatment, benefits in patients with OSAS include improvement of self-reported daytime sleepiness and quality of life (10, 11).
An optimal CPAP level is important. A lower than optimal CPAP level may result in insufficient treatment effects and/or unintentional mask removal, while a higher than optimal CPAP level may induce pressure intolerance and/or mouth dryness (12). Three titration methods are used to establish an optimal level: full-night, split-night, and titration using auto-titrating positive airway pressure (APAP). Of these, full-night, attended titration with polysomnography (PSG) is the gold standard to determine an optimal CPAP level (1). Although full-night manual titration in a sleep laboratory is the preferred method, APAP titration may be performed to identify optimal CPAP level for patients with moderate to severe OSAS without congestive heart failure, chronic obstructive pulmonary disease, or hypoventilation syndrome (13).
Various predictive equations for optimal CPAP level have been developed in several countries and for populations of different geographical origins (14-20). The optimal CPAP level determined by a predictive equation may improve the success rate of manual titration and increase the convenience of treatments such as patient self-titration of CPAP or APAP (21-23). Therefore, the objectives of the present study were: 1) to investigate mean optimal CPAP level according to the severity of OSAS; 2) to examine the factors which affect optimal CPAP level; and 3) ultimately to develop predictive equation for optimal CPAP level using variables which can be easily measured during the diagnostic process in Korean patients with OSAS.
Go to : 
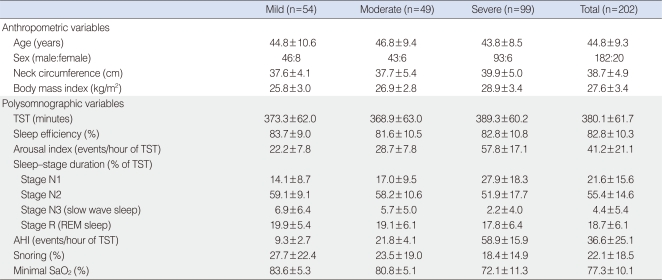
Two hundred two Korean patients diagnosed with OSAS using the apnea-hypopnea index (AHI) in standard PSG ≥5 with signs and symptoms suspicious for OSAS, and who underwent a successful manual titration for CPAP treatment between April 2002 and December 2004 were included in this study. The subjects comprised 182 males and 20 females with mild (n=54), moderate (n=49), and severe (n=99) OSAS (Table 1). Mean age and body mass index (BMI, weight in kg divided by height in m2) was 44.8±9.3 years and 27.6±3.4 kg/m2, respectively. Severity of OSAS was classified according to the degree of AHI. Mild, moderate, and severe OSAS for adults were defined as 5≤AHI<15, 15≤AHI<30, AHI≥30, respectively (24). Neck circumference (cm) was measured at the level just below the most prominent portion of the thyroid cartilage (Adam's apple). The study was reviewed and approved by the Institutional Review Board at Ansan Hospital, Korea University.
All subjects underwent an overnight, in-laboratory attended PSG with an Alice 4 computerized polysomnographic system (Respironics, Atlanta, GA, USA). Sixteen channels were simultaneously used to document the following parameters: four channel electroencephalogram, electrooculogram, submental and leg electromyogram, electrocardiogram, airflow at the nose and mouth (thermistor, nasal pressure transducer), chest and abdominal respiratory movement, oxygen saturation (pulse oximetry), snoring microphone, and body position sensor. A sleep technician observed the behavior of the subjects and confirmed their sleep positions using an infrared camera inside the room. To determine optimal pressure level for CPAP treatment, fullnight, manual titration with the same montage PSG was performed. The optimal pressure level was defined as the lowest effective pressure that controlled most respiratory disturbances including apnea, hypopnea, and snoring in all body positions and in all stages, especially in the supine position during rapid eye movement (REM) sleep.
All the sleep studies were manually interpreted by a sleep technician according to the standard sleep stage and arousal criteria (25, 26). Apnea was defined as absence of airflow for a period lasting at least 10 seconds and hypopnea was defined as at least a 30% reduction in airflow associated with decrease in oxygen saturation of 4% or more. The apnea index was defined as the number of apneic episodes per hour of total sleep time, and the AHI was defined as the number of episodes of apnea and hypopnea per hour of total sleep time. Arousal index was defined as the number of arousals per hour of total sleep time.
All data are presented as the mean±standard deviation for continuous variables, and as frequencies (percent) for categorical variables. Pearson's correlation coefficients were used to analyze relationships between optimal CPAP level and baseline data including anthropometric and polysomnographic variables. Stepwise multiple linear regression analysis was used to select independent predictive variables and to develop a predictive equation for optimal CPAP level. Statistical analysis was performed using SPSS ver. 10.0 (SPSS Inc., Chicago, IL, USA), and P<0.05 was considered statistically significant.
Go to : 
The mean optimal pressure level for CPAP treatment according to the severity of OSAS is shown in Table 2. The mean optimal pressure level in the 202 OSAS patients was 7.8±2.3 cm H2O. The mean optimal pressure levels in the mild, moderate, and severe OSAS groups was 6.0±1.3, 7.4±1.9, and 9.1±2.1 cm H2O, respectively.
Correlations between optimal pressure level for CPAP treatment and baseline variables including demographic and polysomonographic data in the 202 patients are presented in Table 3. AHI (r=0.595, P<0.001), arousal index (r=0.542, P<0.001), minimal SaO2 (r=-0.502, P<0.001), BMI (r=0.494, P<0.001), neck circumference (r=0.265, P<0.001), and age (r=-0.164, P=0.019) were significantly correlated with optimal pressure level.
Of the anthropometric and polysomnographic variables, stepwise multiple linear regression analysis identified BMI and AHI as independent predictive variables associated with the optimal pressure level. The best predictive equation by stepwise regression analysis was:
Optimal CPAP level (cm H2O)=0.681+(0.205×BMI)+(0.040×AHI)
Forty-two percent of the variance in the optimal CPAP level was explained by this equation (R2=0.42, P<0.001).
Go to : 
The ultimate objective of this study, to develop a predictive equation for optimal CPAP level using easily measured variables during diagnostic evaluation in Korean OSAS patients, was accomplished.
Lin et al. (17) constructed a predictive model for optimal CPAP level in 121 adult Taiwanese patients with OSAS and developed the following equation: Optimal CPAP level (cm H2O)=0.52+(0.174×BMI)+(0.042×AHI). Their predictive model is in close agreement with our equation, in that BMI and AHI were the two most important independent predictors of optimal CPAP level. Akahoshi et al. (20) produced a predictive equation derived from anthropometric, polysomnographic, and cephalometric data in 170 Japanese patients with OSAS. In the study, stepwise multiple regression analysis selected BMeH (the angle between a line from point <B> to menton <Me> and a line from Me to the hyoid bone <H>), BMI, AHI, and mean SaO2 as independent predictors of effective pressure. The predictive equation was: Optimal CPAP level (cm H2O)=27.78+(0.041×BMeH)+(0.141×BMI)+(0.040×AHI)-(0.312×mean SaO2). Although optimal pressure was more accurately predicted by combining cephalometric data such as BMeH with BMI, AHI, and mean SaO2, cephalometric analysis may decrease the clinical usefulness of this equation.
Rowley et al. (21) investigated the effect of predictive equations on the success rate of manual titration for CPAP treatment. They found that manual titration using predictive equation modestly increases the success rate of CPAP titration. The success rate of titration studies for which the AHI at the final level tested ≤50% of baseline and ≤10 events/hour increased from 50% to 68% (P<0.001). It is thought that the predictive equation worked by reducing complex steps and time-consuming efforts to determine effective pressure levels. Fitzpatrick et al. (22) compared results between in-laboratory manual titration and home self-titration of CPAP for OSAS in a randomized, singleblind, two-period crossover study. Patient self-titration of CPAP was performed based on the optimal CPAP level by the predictive equation. The authors demonstrated that home self-titration of CPAP using a predictive equation is as effective as in-laboratory manual titration during full-night PSG, with similar CPAP compliance, subjective, and objective outcomes.
Predictive equations for optimal CPAP level may be required for the following objectives: 1) to improve the success rate of full-night, in-laboratory manual titration, because the starting pressure by predictive equation would be closer to the optimal CPAP level (e.g., simplifying the titration process as decreasing numerous pressure changes and time consumption to obtain an optimal pressure level); 2) to increase the convenience and effectiveness of home patient management (e.g., home self-titration of CPAP or APAP); 3) to be substituted for manual titration in several conditions in which manual titration in a sleep laboratory is not possible (e.g., immobility, safety, and critical illness); and 4) as an alternative to manual titration in cases in which titration using a predictive equation would be preferable to manual titration (e.g., high cost, time consumption, and long waiting list).
The strength of the current study is that the study sample was large and included patients with mild OSAS. In two previous East Asian studies, the study samples were 121 patients with moderate to severe OSAS and 170 patients with an AHI of more than 20 (17, 20). CPAP is optionally indicated for the treatment of patients with mild OSAS. We believe that a better predictive equation may be developed for the large number of patients with OSAS, including those with mild OSAS.
There were several limitations in the present study. This study did not include a validation or application of the presently developed equation to another group of Korean OSAS patients. Although cephalometric data may influence optimal pressure level, craniofacial abnormalities were not considered in the development of this predictive equation because the required cephalometric analysis may have diminished the clinical utility of this equation.
In conclusion, a predictive equation for optimal CPAP level was developed using AHI and BMI, which can be easily measured during diagnostic work-up. We expect that this predictive equation for optimal CPAP level will be helpful for designing CPAP treatment in Korean patients with OSAS. CONFLICT OF INTEREST No potential conflict of interest relevant to this article was reported.
Go to : 
ACKNOWLEDGMENTS
This study was supported by a grant of the Korea Healthcare technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A090084).
Go to : 
References
1. Epstein LJ, Kristo D, Strollo PJ Jr, Friedman N, Malhotra A, Patil SP, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009; 6. 15. 5(3):263–276. PMID: 19960649.
2. Kim J, In K, You S, Kang K, Shim J, Lee S, et al. Prevalence of sleep-disordered breathing in middle-aged Korean men and women. Am J Respir Crit Care Med. 2004; 11. 15. 170(10):1108–1113. PMID: 15347562.

3. Powell NB. Contemporary surgery for obstructive sleep apnea syndrome. Clin Exp Otorhinolaryngol. 2009; 9. 2(3):107–114. PMID: 19784401.

4. Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981; 4. 18. 1(8225):862–865. PMID: 6112294.

5. Kim JH, Kwon MS, Song HM, Lee BJ, Jang YJ, Chung YS. Compliance with positive airway pressure treatment for obstructive sleep apnea. Clin Exp Otorhinolaryngol. 2009; 6. 2(2):90–96. PMID: 19565034.

6. Kushida CA, Morgenthaler TI, Littner MR, Alessi CA, Bailey D, Coleman J Jr, et al. Practice parameters for the treatment of snoring and Obstructive Sleep Apnea with oral appliances: an update for 2005. Sleep. 2006; 2. 01. 29(2):240–243. PMID: 16494092.

7. Morgenthaler TI, Kapen S, Lee-Chiong T, Alessi C, Boehlecke B, Brown T, et al. Practice parameters for the medical therapy of obstructive sleep apnea. Sleep. 2006; 8. 01. 29(8):1031–1035. PMID: 16944671.
8. Choi JH, Park YH, Hong JH, Kim SJ, Park DS, Miyazaki S, et al. Efficacy study of a vest-type device for positional therapy in position dependent snorers. Sleep Biol Rhythms. 2009; 7. 7(3):181.

9. Kushida CA, Littner MR, Hirshkowitz M, Morgenthaler TI, Alessi CA, Bailey D, et al. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep. 2006; 3. 01. 29(3):375–380. PMID: 16553024.

10. Ballester E, Badia JR, Hernandez L, Carrasco E, de Pablo J, Fornas C, et al. Evidence of the effectiveness of continuous positive airway pressure in the treatment of sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999; 2. 159(2):495–501. PMID: 9927363.

11. Jenkinson C, Davies RJ, Mullins R, Stradling JR. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet. 1999; 6. 353(9170):2100–2105. PMID: 10382693.

12. Kakkar RK, Berry RB. Positive airway pressure treatment for obstructive sleep apnea. Chest. 2007; 9. 132(3):1057–1072. PMID: 17873201.

13. Morgenthaler TI, Aurora RN, Brown T, Zak R, Alessi C, Boehlecke B, et al. Practice parameters for the use of autotitrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome: an update for 2007. An American Academy of Sleep Medicine report. Sleep. 2008; 1. 01. 31(1):141–147. PMID: 18220088.
14. Miljeteig H, Hoffstein V. Determinants of continuous positive airway pressure level for treatment of obstructive sleep apnea. Am Rev Respir Dis. 1993; 6. 147(6 Pt 1):1526–1530. PMID: 8503565.

15. Sforza E, Petiau C, Weiss T, Thibault A, Krieger J. Pharyngeal critical pressure in patients with obstructive sleep apnea syndrome. Clinical implications. Am J Respir Crit Care Med. 1999; 1. 159(1):149–157. PMID: 9872833.
16. Akashiba T, Kosaka N, Yamamoto H, Ito D, Saito O, Horie T. Optimal continuous positive airway pressure in patients with obstructive sleep apnoea: role of craniofacial structure. Respir Med. 2001; 5. 95(5):393–397. PMID: 11392581.

17. Lin IF, Chuang ML, Liao YF, Chen NH, Li HY. Predicting effective continuous positive airway pressure in Taiwanese patients with obstructive sleep apnea syndrome. J Formos Med Assoc. 2003; 4. 102(4):215–221. PMID: 12833183.
18. Stradling JR, Hardinge M, Paxton J, Smith DM. Relative accuracy of algorithm-based prescription of nasal CPAP in OSA. Respir Med. 2004; 2. 98(2):152–154. PMID: 14971878.

19. Loredo JS, Berry C, Nelesen RA, Dimsdale JE. Prediction of continuous positive airway pressure in obstructive sleep apnea. Sleep Breath. 2007; 3. 11(1):45–51. PMID: 17089145.

20. Akahoshi T, Akashiba T, Kawahara S, Uematsu A, Nagaoka K, Kiyofuji K, et al. Predicting optimal continuous positive airway pressure in Japanese patients with obstructive sleep apnoea syndrome. Respirology. 2009; 3. 14(2):245–250. PMID: 19210648.

21. Rowley JA, Tarbichi AG, Badr MS. The use of a predicted CPAP equation improves CPAP titration success. Sleep Breath. 2005; 3. 9(1):26–32. PMID: 15785918.

22. Fitzpatrick MF, Alloway CE, Wakeford TM, MacLean AW, Munt PW, Day AG. Can patients with obstructive sleep apnea titrate their own continuous positive airway pressure? Am J Respir Crit Care Med. 2003; 3. 01. 167(5):716–722. PMID: 12598214.

23. Masa JF, Jimenez A, Duran J, Capote F, Monasterio C, Mayos M, et al. Alternative methods of titrating continuous positive airway pressure: a large multicenter study. Am J Respir Crit Care Med. 2004; 12. 01. 170(11):1218–1224. PMID: 15282204.
24. Kushida CA, Littner MR, Morgenthaler T, Alessi CA, Bailey D, Coleman J Jr, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005; 4. 01. 28(4):499–521. PMID: 16171294.

25. Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. 1968. Bethesda, MD: U. S. National Institute of Neurological Diseases and Blindness, Neurological Information Network.
26. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992; 4. 15(2):173–184. PMID: 11032543.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download