Abstract
Objectives
While other cytokines are known to be associated with otitis media with effusion (OME), the involvement of heat shock protein 70 (HSP70) in middle ear effusion (MEE) is unknown. This study was undertaken to investigate the possibility of there being a HSP70 expression in human MEE and to determine its potential role as a cytokine in OME.
Methods
The levels of HSP70, tumor necrosis factor-alpha and interleukin-1beta were measured by enzyme-linked immunosorbent assay in the effusion of different groups of OME patient following collection of the MEE using our new collection system. The clinical characteristics of the OME patients and the MEE status were analyzed.
Results
HSP70 was expressed in all the types of MEE. The mucous and seromucous effusions showed higher HSP70 levels than that of the serous effusion. The HSP70 level was correlated with the levels of tumor necrosis factor (TNF)-α and interleukin (IL)-1β in the effusions. The positive correlations between HSP70, TNF-α and IL-1β were statistically significant (P<0.05).
Otitis media with effusion (OME) is a disease that's characterized by the presence of fluid in the middle ear. Despite the substantial investigation that has been done on OME, the precise etiology and pathogenesis of OME still remains unclear. Immune disturbances and Eustachian tube dysfunction are considered to be most important contributing factors. Several cytokines, including interleukin-1beta (IL-1β), IL-8 and tumor necrosis factor-alpha (TNF-α), and complement C3 are considered to play key roles in the initiation and maintenance of the inflammatory response in OME (1-3). Other allergy-related pathomechanisms may underlie OME. The Th-2-driven cytokines IL-4, IL-6, and TNF-α may contribute to the allergy-related persistence of OME (4). More knowledge concerning the pathomechanisms of OME and the contributing cytokines is needed to develop truly efficacious treatment and prevention strategies.
Heat shock proteins (HSPs) are essential for the survival of cells that are undergoing environmental stress. Various families of HSPs, including HSP90, HSP70, HSP60, and HSP27, have been categorized based on their molecular weights and functional attributes (5). Their synthesis is enhanced by exposure to a variety of detrimental conditions such as heat, toxins, oxidative stress and glucose deprivation (6). HSP70 is expressed in the middle ear mucosa of experimentally induced acute otitis media (AOM) (7, 8). Even though it has been considered that HSPs mainly function as molecular chaperones, they also appear to be involved in diverse biological activities such as apoptosis, carcinogenesis and cytoprotection from cytotoxic damage. The cytoprotective role of HSP 70 and the relationship between HSP70 and inducible nitric oxide synthase (iNOS) has recently been studied in gastritis induced by the pathogenic bacterium helicobacter pylori (9). The latter study is intriguingly germane to otolaryngology because of the similar pathomechanisms that are involved in the iNOS pathway. In another study, the proteomic analysis of murine skin demonstrated that HSP70 is an important mediator of allergic contact hypersensitivity, which indicates that inhibiting its activity may be beneficial for preventing autoimmune and immunological hypersensitivity diseases (10).
Considering its confounding effect on various inflammatory processes, HSP70 may well be important in the pathoetiology of diseases that affect different organs, or at different stages of a disease. To the best of our knowledge, there have been no published reports concerning the expression and the possible role of HSP70 in the middle ear effusion (MEE) of humans during the disease process of OME. Here we report for the first time on the expression of HSP70 in human MEE and its possible role in the chronicity of OME as an inflammatory mediator or indicator.
A total of 30 ears from children aged 9 months to 15 yr (mean age, 4.1±3.6 yr) and who had chronic OME for more than 2 months (range, 2 to 10 months) and 38 ears from adults who were medically treated and observed for their OME for at least 1 month and who were undergoing myringotomy or tympanotomy tube placement at Seoul St. Mary's Hospital were included in this study. The study has been approved by the Institutional Review Board of the Catholic Medical Center (Seoul St. Mary's Hospital). The adults were subgrouped according to their previous history of head and neck region radiation therapy (received or not received) as their concomitant cancer treatment to observe the difference in the cytokine and HSP70 expressions in the MEE between the two groups.
To collect the various types of the MEE without any loss, we designed a MEE sampling system that consisted of a 1 cc tuberculin syringe, a long and thin intravenous (i.v.) catheter and different sizes of blunt spinal needles (17-23 G), as shown in Fig. 1. The amount of the effusion was directly measured within the syringe. The nature of the fluid (serous, mucous or seromucous) was determined by the attending surgeon. Serum-like yellowish clear fluid without any viscosity was considered as serous, and thick viscous fluid that was hard to collect was considered as mucous. The fluid that contained both of serous and mucous components was considered as seromucous. The collected MEE was stored at -70℃ until it was used.
The clinical data concerning OME was obtained from the participants by using a specially designed questionnaire. The information obtained included the frequency of tympanostomy tube insertion, the duration of OME and the allergy history of each patient. An audiology test measuring the pure tone averages of the air and bone conduction hearing levels at the speech frequency was performed preoperatively for most of the patients.
The total protein was assayed using a Bradford-based commercial kit (BioRad, Hercules, CA, USA) with bovine serum albumin as a standard. The TNF-α, IL-1β and HSP70 levels in the MEE were determined using a specific enzyme-linked immunosorbant assay (ELISA) kit for human TNF-α (Biosource, Camarillo, CA, USA), human IL-1β (R&D Systems, Minneapolis, MN, USA) and HSP70 (Stressgen Biotechnologies, Victoria, BC, Canada) according to the instructions of the particular manufacturer.
One-way ANOVA, Chi-square tests and standard Pearson correlation tests were used to compare the clinical data, the hearing levels, the nature of the fluid, the total protein and the TNF-α, IL-1β and HSP70 levels in the different groups. Statistical analyses were done using SPSS ver. 11.5 (SPSS Inc., Chicago, IL, USA). Statistical significance was set at P-values < 0.05.
The patients were 30 children (mean age, 4.1 yr), 27 adults who had not received radiation therapy (mean age, 62.5 yr) and 11 adults who had received radiation therapy (mean age, 58 yr). The basic demographic composition of these study groups is shown in Table 1. The gender distribution, the duration of OME and the hearing levels among the three different groups were not significantly different. The number of previous tympanostomy tube insertions was higher for the adults who had not received radiation therapy. Almost all the effusions in the children were mucous or seromucous in nature, whereas most of the adult effusions were serous. These observations are consistent with those previously reported for adult OME (11, 12).
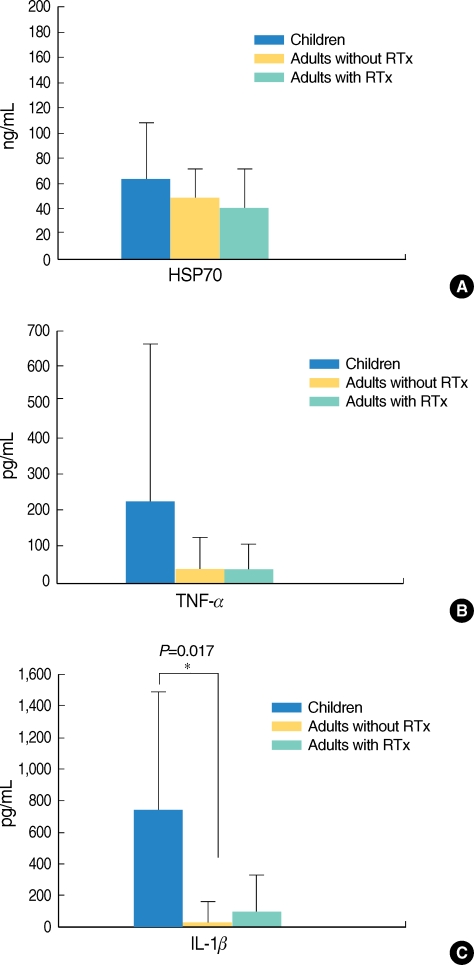
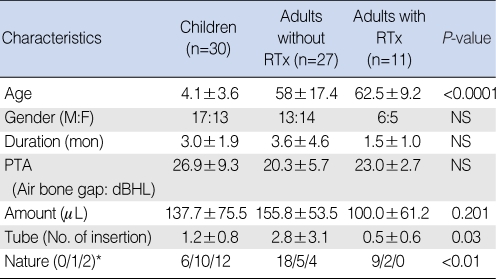
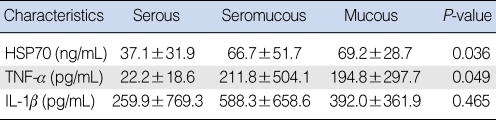
HSP70 was detected in all the MEEs in the three study groups, but the level varied quantitatively (64±44 ng/mL for the children, 48±32 ng/mL for the adults who had not received radiation therapy and 41±33 ng/mL for the adults who had received radiation therapy). The quantitative differences were not statistically significant (Fig. 2A). The levels of TNF-α in the different groups were also analyzed. The average concentration of TNF-β in the MEE was 232±453 pg/mL for the children, 44±84 pg/mL for the adults who had not received radiation therapy and 42±47 pg/mL for the adults who had received radiation therapy (Fig. 2B). The higher TNF-α level in children was not statistically significant (P=0.055). The IL-1β MEE level was highest in the children (743±931 pg/mL), followed by that in the adults who had received radiation therapy (114±248 pg/mL) and that in the adults who had not received radiation therapy (38±88 pg/mL). The latter's IL-1β MEE level was significantly different from that of the children (P<0.05). No other statistically significant differences between the groups were evident for IL-1β (Fig. 2C).
The levels of HSP70 in the mucous and seromucous effusions were significantly higher than that of the serous effusion (P<0.05). A similar phenomenon was observed for the levels of TNF-α in the MEE (P<0.05). However, the IL-1β levels did not show any meaningful differences in the different types of effusion (Table 2).
The HSP70 level was correlated with the levels of TNF-α (r=0.48) and IL-1β (r=0.31) in the effusions. The highest correlation was between two early cytokines(TNF-α and IL-1β) (r=0.62). The positive correlations between HSP70, TNF-α and IL-1β were statistically significant (P<0.05). There was no correlation evident between the level of HSP70 and age, but negative correlations were evident between TNF-α/IL-1β and age. The levels of all three cytokines were not significantly correlated with the amount of MEE (Table 3).
HSPs are crucial for the maintenance of cell integrity during both normal cell growth and pathophysiological conditions (13). Their expressions are induced by various conditions such as heat, oxidative stress and drug exposure; the type of HSP induced and its level of expression are responsible for the fate of a cell in response to stress or stimulus (14). HSP70 is expressed during the inflammatory process of experimentally induced MEE by bacterial inoculation (7). However, there has been no report regarding the HSP70 expression or its quantification in human OME. Yet given that this protein has been detected in the middle ear mucosa of an experimental model of AOM (7), we hypothesized that HSP70 could be expressed in the mucosa of the middle ear during the disease process of human OME, and it could be secreted from the inflamed mucosa to the MEE. The MEE HSP70 levels in the current study were measured using ELISA and the level of this protein was compared to other well-known early onset cytokines such as TNF-α and IL-1β in the MEEs. The levels of HSP70 in human MEE and their correlations with the nature of the effusion and the other clinical characteristics have not been previously reported on.
For the proper collection of MEE, we developed the collection system (Park's MEE collection system) shown in Fig. 1. This sampling system is easy to assemble in the hospital setting. Moreover, its long, thin i.v. catheter connected to the back side of a 1 mL tuberculin syringe enabled controlling the suction power, which prevents loss of the MEE. A third advantage is the various sized blunt spinal needles that are directly connected to the tuberculin syringe, which eases the collection of different types of MEE, including the fluid that is thick and mucous in nature. The direct collection of MEE using the graded tuberculin syringe provides an easy, accurate means of measuring the volume of the recovered MEE. Finally, the collected MEE can be directly stored in the syringe, which lessens the possibility of contamination or the loss of the MEE.
The present study reports the first description of the HSP70 expression in human MEE. Indeed, HSP70 was detected in the mucous, seromucous and serous MEEs. The higher level detected in the mucous and seromucous effusions, as compared to the serous effusion, suggests that HSP70 should be considered as another cytokine that may be related with the chronicity of OME. HSPs are crucial for the maintenance of cell integrity during both normal cell growth and pathophysiological conditions (13). They also play a cytoprotective role in other mucosal tissues. For example, those guinea pig gastric mucosal cells in which HSPs have been induced by heat shock treatment can show considerable resistance to injury induced by ethanol (15). The cytoprotective mechanism of HSPs in various stressful conditions has been revealed by modulating the expression of an enzyme that causes tissue damage. In rat pulmonary artery smooth muscle cells, the expression of IL-1β-induced iNOS can be inhibited by a preinduced HSP70 expression (16). After challenge with endotoxin, the induced HSP 70 expression in the mesenteric artery, lung and liver can significantly attenuate hypotension and it can up-regulate the iNOS m RNA expression in rats (17). iNOS is expressed in the middle ear mucosa when the inflammatory reaction is initiated by various types of insults (18, 19), and iNOS is also induced by early onset cytokines, including IL-1β and TNF-α, in a MEE. The latter inductions might be related to the cytoprotective role of HSP70 in OME, as was shown in other tissues.
From the beginning of this study, we hypothesized that the MEE in different age groups (adults and children) and in the groups with a definitive causal factor such as Estachian tube dysfunction after radiation therapy (adults with RT) or without a definitive causal factor (adults without RT) might show different cytokine levels and HSP70 levels. We found the difference of the early cytokine expressions in the different age groups, but we could not find any difference of the early cytokine expressions in the two different adult groups. We could conclude that the disease process of MEE in adults and children seemed to be different, whereas the MEE in adults with or without RT did not seem to have a different inflammatory process. Since there is well known longevity of the disease process from the serous effusion to the seromucous infusion to the mucous effusion in OME, we can postulate that the nature of the fluid might represent the chronicity of the disease here in our study. The result of our study showed that HSP70 and TNF-α were highly expressed in the mucous MEE, and that the HSP70 level was positively correlated with the levels of TNF-α and IL-1β. In the previous study by Nell and Grote (20), early cytokines such as TNF-α and IL-1β were expressed at a higher level in the mucous effusion than that in the in serous effusion, which was the same result as ours. They concluded that the thick and mucoid effusions are an indication of chronic disease. We could not find any significant difference in the HSP70 level according to the age group. These results support the contention that the HSP70 in MEE could be a cytokine that is secreted from the middle ear mucosa and it is a good indicator of disease chronicity. Considering the positive correlation between HSP70 and TNF-α/IL-1β we can also postulate a possible representative role for HSP70 as a cytoprotectant of the middle ear mucosa during OME. We can suggest the possible pathomechanism of the HSP70 expression in the MEE during the disease process of OME and its relationship with the other early cytokines. During the early stage of OME that is evident as serous effusion, generation and secretion of inflammatory mediators, including TNF-α and IL-1β might be low and so this would not lead to a high expression of HSP70 in the middle ear mucosa. As the disease progresses, the higher generation of the inflammatory mediators in the middle ear mucosa would lead to the higher expression of HSP70 in the middle ear epithelial cells and its secretion into the MEE. Future experiments designed to investigate the iNOS expression in MEE, which might be related to the expression of HSP70, could be helpful for understanding the disease process of OME. Further studies to investigate the more precise functional role of HSP70 in OME will be necessary to provide clues regarding other perspectives on the pathomechanism and treatment of OME.
We performed this study to investigate the possibility of an HSP 70 expression in human MEE and to examine its potential role as a cytokine during the disease process of OME. The study results show that HSP70 is expressed in the various types of human MEE and the HSP70 level is positively correlated with the levels of other cytokines in MEE. As far as we can determine, this is the first report on the HSP70 expression in human MEE. The highly elevated level of HSP70 in the seromucous and mucous effusions indicates that this protein might be related with the chronicity of this disease. The specially designed MEE collection system (Park's MEE collection system) that was successfully used during this study should prove to be valuable when conducting future studies on human MEE.
ACKNOWLEDGEMENTS
This work was supported by a grant from the alumni of the Department of Otolaryngology, the Catholic University of Korea School of Medicine, Seoul, Korea. This paper has been represented at the 6th Extraordinary International Symposium on the Recent Advances in Otitis Media Research in Seoul, May 2009.
References
1. Yellon RF, Doyle WJ, Whiteside TL, Diven WF, March AR, Fireman P. Cytokines, immunoglobulins, and bacterial pathogens in middle ear effusions. Arch Otolaryngol Head Neck Surg. 1995; 8. 121(8):865–869. PMID: 7619411.

2. Maxwell KS, Fitzgerald JE, Burleson JA, Leonard G, Carpenter R, Kreutzer DL. Interleukin-8 expression in otitis media. Laryngoscope. 1994; 8. 104(8 Pt 1):989–995. PMID: 8052085.

3. Narkio-Makela M, Teppo AM, Meri S. Complement C3 cleavage and cytokines interleukin-1beta and tumor necrosis factor-alpha in otitis media with effusion. Laryngoscope. 2000; 10. 110(10 Pt 1):1745–1749. PMID: 11037838.
4. Jang CH, Kim YH. Characterization of cytokines present in pediatric otitis media with effusion: comparison of allergy positive and negative. Int J Pediatr Otorhinolaryngol. 2002; 10. 21. 66(1):37–40. PMID: 12363420.

5. Trautinger F. Heat shock proteins in the photobiology of human skin. J Photochem Photobiol B. 2001; 10. 63(1-3):70–77. PMID: 11684453.

6. Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol. 2002; 3. 2(3):185–194. PMID: 11913069.

7. Egusa K, Huang CC, Haddad J Jr. Heat shock proteins in acute otitis media. Laryngoscope. 1995; 7. 105(7 Pt 1):708–713. PMID: 7603274.

8. Bi AF, Zou J, Zhou YQ, Shang YD, Zhao QS. HSP70 expression of middle ear mucosa in acute suppurative otitis media. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2000; 4. 14(4):173–174. PMID: 12541495.
9. Yeo M, Park HK, Kim DK, Cho SW, Kim YS, Cho SY, et al. Restoration of heat shock protein70 suppresses gastric mucosal inducible nitric oxide synthase expression induced by Helicobacter pylori. Proteomics. 2004; 11. 4(11):3335–3342. PMID: 15378740.
10. Yusuf N, Nasti TH, Huang CM, Huber BS, Jaleel T, Lin HY, et al. Heat shock proteins HSP27 and HSP70 are present in the skin and are important mediators of allergic contact hypersensitivity. J Immunol. 2009; 1. 01. 182(1):675–683. PMID: 19109201.

11. Morton RP, Woollons AC, McIvor NP. Nasopharyngeal carcinoma and middle ear effusion: natural history and the effect of ventilation tubes. Clin Otolaryngol Allied Sci. 1994; 12. 19(6):529–531. PMID: 7895386.

12. Ondrey FG, Juhn SK, Adams GL. Early-response cytokine expression in adult middle ear effusions. Otolaryngol Head Neck Surg. 1998; 10. 119(4):342–345. PMID: 9781987.

13. Takayama S, Reed JC, Homma S. Heat-shock proteins as regulators of apoptosis. Oncogene. 2003; 12. 08. 22(56):9041–9047. PMID: 14663482.

14. Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002; 3. 08. 295(5561):1852–1858. PMID: 11884745.

15. Nakamura K, Rokutan K, Marui N, Aoike A, Kawai K. Induction of heat shock proteins and their implication in protection against ethanol-induced damage in cultured guinea pig gastric mucosal cells. Gastroenterology. 1991; 7. 101(1):161–166. PMID: 2044905.

16. Wong HR, Finder JD, Wasserloos K, Pitt BR. Expression of iNOS in cultured rat pulmonary artery smooth muscle cells is inhibited by the heat shock response. Am J Physiol. 1995; 12. 269(6 Pt 1):L843–L848. PMID: 8572246.

17. Hauser GJ, Dayao EK, Wasserloos K, Pitt BR, Wong HR. HSP induction inhibits iNOS mRNA expression and attenuates hypotension in endotoxin-challenged rats. Am J Physiol. 1996; 12. 271(6 Pt 2):H2529–H2535. PMID: 8997314.

18. Kang JM, Yeo SW, Chang KH, Suh BD. Expression of inducible nitric oxide synthase in the experimental otitis media with effusion. Korean J Otolaryngol - Head Neck Surg. 1999; 12. 42(12):1483–1489.
19. Li W, Lin J, Adams GL, Juhn SK. Expression of inducible nitric oxide synthase (iNOS) in middle ear epithelial cells by IL-1beta and TNF-alpha. Int J Pediatr Otorhinolaryngol. 2000; 9. 19. 55(2):91–98. PMID: 11006448.
20. Nell MJ, Grote JJ. Endotoxin and tumor necrosis factor-alpha in middle ear effusions in relation to upper airway infection. Laryngoscope. 1999; 11. 109(11):1815–1819. PMID: 10569413.
Fig. 1
Our middle ear effusion collection system (Park's MEE collection system). This system, which was designed as part of the current study, consists of a 1 mL tuberculin syringe, an 18-23 gauge blunt spinal needle and an intravenous catheter. The backside of the syringe was connected to the catheter, and the catheter was then connected to a suction tube, and the system prevents any loss of effusion.

Fig. 2
Comparison of heat shock protein (HSP)70, tumor necrosis factor (TNF)-α and interleukin (IL)-1β in the different groups with otitis media with effusion. (A) HSP70 was expressed in all the types of middle ear effusion in the three groups. The HSP70 levels were 64±44 ng/mL in the children, 48±32 ng/mL in the adults who did not receive radiation therapy and 41±33 ng/mL in the adults who did receive radiation therapy. The level of HSP70 in the middle ear effusion (MEE) of the children was slightly higher than those levels of HSP70 of the adult groups, although the difference was not significant. (B) The level of TNF-α in the MEE of the children was higher than those TNF-α levels of the adults, although the difference was not statistically significant (P=0.055). (C) The level of MEE IL-1β in the children was significantly different from that in the adults who had not received radiation treatment (P<0.05). RTx: radiation therapy.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download