Abstract
A schwannoma of the larynx is a rare benign tumor that usually presents as a submucosal mass in the pyriform sinus and the aryepiglottic space, and this type of schwannoma constitutes a diagnostic and therapeutic challenge for otolaryngologists. We present here two cases of supraglottic schwannomas that were misdiagnosed as laryngoceles. Both were excised through a lateral thyrotomy approach without a tracheostomy, and the laryngeal function was successfully maintained. We discuss the clinical and imaging findings and the management of this rare neoplasm with focusing on the differential diagnosis of laryngeal schwannoma and laryngocele. We also review the relevant medical literature.
A schwannoma is a benign tumor that originates from the Schwann cells that sheathen the nerve fibers. Neurogenic tumors rarely involve the larynx and they constitute only 0.1-1.5% of all benign laryngeal tumors (1). Laryngeal schwannomas usually arise from the false vocal cord or the aryepiglottic fold. We experienced two supraglottic schwannomas that were initially misdiagnosed as laryngoceles. Both were successfully excised through a lateral thyrotomy approach without a tracheostomy.
A 55-years-old woman presented with the sensation of a foreign body in her throat and hoarseness that had worsened for the 3 months before her presentation. Her occupation was a house keeper. Laryngoscopy revealed a submucosal bulging mass at the left pyriform sinus and this mass extended to the ipsilateral false vocal fold; the left vocal fold was pushed medially and the shape of the mass did not alter with respiration (Fig. 1A). A soft-tissue density mass was observed at the level of the glottis on a lateral C-spine X-ray. Computed tomography (CT) showed a 2-cm mass that contained ring-like calcification, and the mass was located in the left supraglottic area (Fig. 2A). A well-encapsulated, solid mass was detected when performing laryngomicroscopic surgery (LMS). The frozen biopsy was reported as a schwannoma. Owing to the extensive range and fixation of the mass, we had to conduct a second operation via an external approach to achieve complete removal. After the patient was first discharged, magnetic resonance imaging (MRI) confirmed the presence of a well-defined ovoid mass with isointensity on the T1-weighted images and high signal intensity on the T2-weighted images (Fig. 2). We excised the mass via a lateral thyrotomy without a preliminary tracheostomy in the second operation. A window was created using an electrical saw. After dissecting the soft tissues, the yellow mass was easily separated from the adjacent tissues with finger dissection. The internal branch of the superior laryngeal nerve was found to be the nerve of origin for the tumor. No postoperative complications occurred, and she was discharged on postoperative forth day. Postoperative laryngoscopy, which was performed 4 months after the second discharge, revealed that the bulging findings of the left vocal fold had disappeared and both vocal folds were mobile. The lady's voice recovered completely.
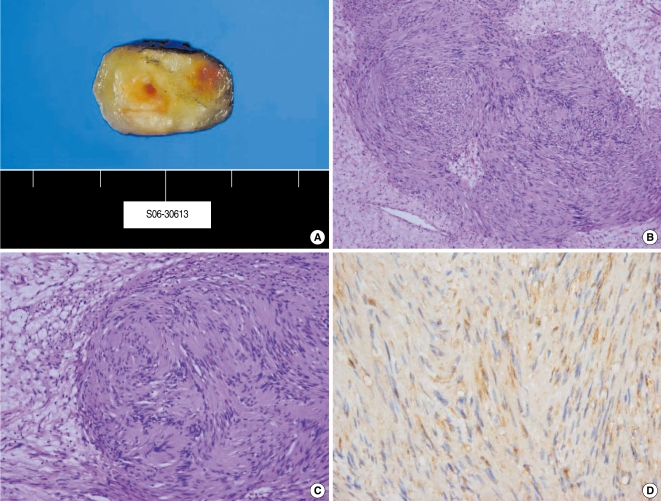
The gross tumor specimen measured 2.0×2.5 cm. On microscopic examination, the tumor was characterized by cellular Antoni A areas alternating with myxoid, loose degenerative Antoni B type areas. The immunohistochemical study was positive for S-100 protein in the cellular areas. The overall features of the tumor were consistent with a schwannoma.
A 47-years-old man visited our clinic with complaints of a foreign body sensation and hoarseness that had begun 18 months earlier. He worked as an office worker. On direct laryngoscopy, there was a submucosal bulging mass at the left ventricle and the shape of the mass did not change during phonation (Fig. 1B). CT showed a 2.2×1.7 cm benign-looking mass in the left larynx and at the supraglottic and glottic levels (Fig. 2B). We performed LMS under the working diagnosis of laryngocele. Complete excision was impossible because of the hard, fixed nature of the mass. We finished the operation and planned to subsequently remove the mass at a later date via a lateral thyrotomy approach. One month after the patient was first discharged, diagnostic imaging revealed a highly enhanced mass on the T2-weighted MRI with delayed enhancement on the T1-weighted MRI. The differential diagnosis was a schwannoma or hemangioma (Fig. 2B). Two months after the LMS, we performed a lateral thyrotomy without a preliminary tracheotomy. A round encapsulated mass was identified after dissecting the soft tissues and it was easily separated without injury to the adjacent tissues. Several holes were drilled around the window. Using nylon, the window was pulled through the holes and primarily closed (Fig. 3). The patient's dyspnea and dysphagia improved on the first postoperative day. After 2 months, the man's voice became better than before, but some hoarseness still remained. The left vocal fold showed a bowing feature on laryngoscopy during phonation. The laryngeal nerve was noted to be intact on the laryngeal electromyography. So this bowing feature seemed to be an acute postoperative change and we have followed up the patient to observe his voice on an OPD basis.
The gross tumor specimen measured 2.2×1.7 cm. The cut tumor section was yellow. On microscopic examination, the tumor was characterized by cellular Antoni A areas alternating with myxoid, loose degenerative Antoni B type areas. Immunohistochemically, the cellular areas were positive for S-100 protein (Fig. 4).
These two cases indicated that patients with laryngeal schwannomas could present with characteristic symptoms that were quite similar to those of laryngoceles. If we had considered laryngeal schwannoma as part of the differential diagnosis, we could have avoided unnecessary anesthesia and surgery. Therefore, otolaryngologists should choose appropriate diagnostic methods and surgical approaches when a patient presents with a laryngeal mass. Otolaryngologists should be familiar with the following characteristics of laryngeal schwannomas and larngoceles.
Laryngoceles are the most frequent submucosal laryngeal masses and they account for approximately 5% of all benign laryngeal lesions (2, 3). They may be filled with air, fluid or purulent material. Theoretically, a laryngocele is filled with air and a saccular cyst is filled with fluid, but many physicians use the term laryngocele for both. The cause of laryngoceles is unclear, but it is probably related to increased intralaryngeal pressure. It may also occur secondary to inflammation or neoplastic lesions that occur around the laryngeal ventricle, and this can cause a flap-valve mechanism and allow air to enter, but not exit the saccule. There is an association between laryngoceles and laryngeal carcinoma (4). Squamous cell carcinoma of the larynx is the most common tumor accompanying laryngoceles, but there have been no reports of laryngoceles occurring because of a laryngeal schwannoma.
A schwannoma is a benign nerve sheath neoplasm that originates from Schwann cells. Laryngeal schwannomas are very rare, and they account for less than 0.1-0.5% of all the benign neoplasms of the larynx (5). They are encapsulated and they grow eccentrically away from the nerve trunk. The internal branch of the superior laryngeal nerve is the most likely nerve of origin (6). The final diagnosis is based on histologically demonstrating characteristic spindle cells with typical nuclear palisading, Antoni A and B areas on the microscopic examination and positive immunohistochemistry for S100 protein. The Antoni A pattern is characterized by compact, spindle-shaped cells whose nuclei occasionally line up in palisades. Antoni B describes loosely arranged spindle cells within a myxoid matrix that is prone to degeneration. All three of these features were seen in both of our cases.
The symptoms and findings of both laryngoceles and laryngeal schwannomas are very similar. The major symptoms are odynophagia, dysphasia, dyspnea, hoarseness, a globus sensation and stridor (7, 8). On laryngoscopy, a submucosal bulge arising from the false vocal cord or the aryepiglottic fold can be seen along with both of them (5, 9-11). So, the symptoms and laryngoscopic findings can not provide adequate information to distinguish these two diseases in many cases.
An image study is usually helpful for differentiating laryngeal schwannomas from laryngoceles. On CT, a schwannoma appears as a homogenous, isodense, enhancing mass. Tumors larger than 3 cm often show heterogenic density on contrast enhancement with central low attenuation, and this is surrounded by a peripheral enhancing ring (12). Most laryngoceles appear as having air to near-water density or as a soft-tissue density depending on the nature and consistency of their contents, and this characteristic finding helps us to easily differentiate laryngoceles from other submucosal lesions (11). However, a laryngocele containing mucoid or purulent contents can be seen as homogeneously hypodense on non-contrast CT. An infected laryngocele may also have peripheral rim enhancement (8). In these cases, the information from a CT scan can be indistinct.
MRI can be useful for making a diagnosis when the information from CT is insufficient. A mucoid-filled laryngocele has a low signal on the T1-weighted images and it is hyperintense on the T2-weighted images (8). By contrast, a schwannoma occasionally appears iso- to hyperintense on the T1-weighted images and it is mostly highly enhanced on the T2-weighted images (13). Thus, the difference in the signal on the T1-weighted images between a laryngocele and a laryngeal schwannoma is helpful for making the proper diagnosis.
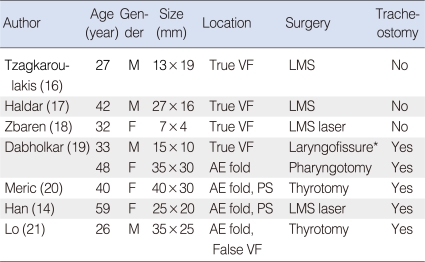
Complete excision is the treatment of choice for both laryngeal schwannomas and laryngocele. For successful treatment, preserving the laryngeal function and mucosal coverage of the exposed cartilage is vital. Laryngoceles are usually removed endoscopically by using a laser. For the cases of laryngeal schannoma, the surgical approach can involve the endoscopic or external approaches, and the final decision is influenced by the size and location of the neoplasm, the damage to the structure of the larynx, the surgeon's preference and the risk of mucosal injury. Variant surgical approaches according to size and location had been reported (Table 1). Size is usually the major determinant of the surgical method. Most reports have suggested performing endoscopic excision for small lesions and an external approach for large ones. Some of them has suggested an external approach for even a small lesion that is located at the aryepiglottic fold and pyriform sinus (14). The need for a preliminary tracheostomy during the surgical removal of a schwannoma of the larynx is controversial. Several studies have recommended performing a preliminary tracheostomy for large lesions to prevent airway obstruction resulting from laryngeal swelling, but there are no standard indications. For our cases, one tumor was 2.5 cm in size and the other was 2.1 cm in size. The size did not clearly indicate which surgical approach to choose, while the location (the pyrifrom sinus) was unfavorable for endoscopic removal, as more mucosal injury was expected due to the poor surgical field. Extensive mucosal injury could increase the risk of intraoperative hemorrhage, wound swelling and postoperative dyspnea. While, a lateral thyrotomy incurs less risk to the voice and it can provide adequate exposure and superb visibility of the paraglottic space (15). So this approach was thought to be the best management for our cases, and consequently both tumors could be successfully removed through a lateral thyrotomy without a preliminary tracheostomy. Therefore, the surgical approach to a laryngeal schwannoma should be determined after considering both its size and location. A preliminary tracheostomy may not be required if the findings do not suggest that further wound swelling.
Schwannomas of the larynx are very rare, but they should be considered in the differential diagnosis of a submucosal laryngeal mass. CT or MRI is useful for determining the extent of this lesion. In our experience both, the location and size of the schwannoma are important factors for determining the surgical approach. A preliminary tracheostomy is not necessary when minimal wound swelling is expected.
References
1. Jones SR, Myers EN, Barnes L. Benign neoplasms of the larynx. Otolaryngol Clin North Am. 1984; 2. 17(1):151–178. PMID: 6326016.

2. De Foer B, Hermans R, Van der Goten A, Delaere PR, Baert AL. Imaging features in 35 cases of submucosal laryngeal mass lesions. Eur Radiol. 1996; 6(6):913–919. PMID: 8972332.

3. Newman BH, Taxy JB, Laker HI. Laryngeal cysts in adults: a clinicopathologic study of 20 cases. Am J Clin Pathol. 1984; 6. 81(6):715–720. PMID: 6731351.

4. Szymczyk K, Kubik P, Misiolek M. A case of laryngocele with laryngeal carcinoma. Nowotwory. 1990; Oct–Dec. 40(4):284–286. PMID: 2277780.
5. al-Otieschan AT, Mahasin ZZ, Gangopadhyay K, al-Dayel F, Jamshed A. Schwannoma of the larynx: two case reports and review of the literature. J Otolaryngol. 1996; 12. 25(6):412–415. PMID: 8972436.
6. Nanson EM. Neurilemoma of the larynx: a case study. Head Neck Surg. 1978; Sep–Oct. 1(1):69–74. PMID: 756397.

7. Cadoni G, Bucci G, Corina L, Scarano E, Almadori G. Schwannoma of the larynx presenting with difficult swallowing. Otolaryngol Head Neck Surg. 2000; 5. 122(5):773–774. PMID: 10793366.

8. Alvi A, Weissman J, Myssiorek D, Narula S, Myers EN. Computed tomographic and magnetic resonance imaging characteristics of laryngocele and its variants. Am J Otolaryngol. 1998; Jul–Aug. 19(4):251–256. PMID: 9692634.

9. Stanley RJ, Scheithauer BW, Weiland LH, Neel HB 3rd. Neural and neuroendocrine tumors of the larynx. Ann Otol Rhinol Laryngol. 1987; Nov–Dec. 96(6):630–638. PMID: 2825582.

10. Yousem DM, Oberholtzer JC. Neurofibroma of the aryepiglottic fold. AJNR Am J Neuroradiol. 1991; Nov–Dec. 12(6):1176–1178. PMID: 1763745.
11. Danish HM, Meleca RJ, Dworkin JP, Abbarah TR. Laryngeal obstructing saccular cysts: a review of this disease and treatment approach emphasizing complete endoscopic carbon dioxide laser excision. Arch Otolaryngol Head Neck Surg. 1998; 5. 124(5):593–596. PMID: 9604989.
12. Plantet MM, Hagay C, De Maulmont C, Mahe E, Banal A, Gentile A, et al. Laryngeal schwannomas. Eur J Radiol. 1995; 11. 21(1):61–66. PMID: 8654462.

13. Akeson P, Holtas S. Radiological investigation of neurofibromatosis type 2. Neuroradiology. 1994; 36(2):107–110. PMID: 8183445.

14. Han MW, Kim IJ, Nam SY. A case of schwannoma of the larynx. Korean J Otorhinolaryngol-Head Neck Surg. 2008; 3. 51(3):289–291.
15. Thome R, Thome DC, De La Cortina RA. Lateral thyrotomy approach on the paraglottic space for laryngocele resection. Laryngoscope. 2000; 3. 110(3 Pt 1):447–450. PMID: 10718436.
16. Tzagkaroulakis A, Stivaktakis J, Nikolopoulos T, Davilis D, Zervoudakis D. Ancient schwannoma of the true vocal cord. ORL J Otorhinolaryngol Relat Spec. 2003; Sep–Oct. 65(5):310–313. PMID: 14730191.

17. Haldar A, Choudhury A, Banerjee P, Das S, Sinha R. Schwannoma of larynx: a rare presentation. Indian J Otolaryngol Head Neck Surg. 2008; Jan–Mar. 60(1):53–55.
18. Zbaren P, Markwalder R. Schwannoma of the true vocal cord. Otolaryngol Head Neck Surg. 1999; 12. 121(6):837–839. PMID: 10580252.

19. Dabholkar JP, Chhabria S, Mishra M, Chirmade S, Sikdar A. Benign laryngopharyngeal lesions: a case series. Indian J Otolaryngol Head Neck Surg. 2008; Jan–Mar. 60(1):7–10.

20. Meric F, Arslan A, Cureoglu S, Nazaroglu H. Schwannoma of the larynx: case report. Eur Arch Otorhinolaryngol. 2000; 12. 257(10):555–557. PMID: 11195036.
21. Lo S, Ho WK. Schwannoma of the larynx: an uncommon cause of vocal cord immobility. Hong Kong Med J. 2004; 4. 10(2):131–133. PMID: 15075434.
Fig. 1
The preoperative laryngsocpic findings. In case 1, there was a bulging mass noted at the left pyriform sinus on laryngoscopy (A). In case 2, a bulging mass at the left false vocal fold was seen (B).

Fig. 2
Neck CT and MRI of the laryngeal schwannomas. In case 1, about a 2cm sized low density lesion in the left supraglottic larynx was seen and the lesion contained a small ring-like calcific density. The MRI scan showed an iso-intense signal on the T1-weighted image and a well defined border in the larynx and minimal contrast enhancement on the T2-weighted image (A). In case 2, a 2.2×1.7 cm sized benign-looking mass was noted in the left larynx and the mass showed heterogenic density with focal enhancement on the CT scan. The T1-weighted image showed an iso-intense signal and the T2-wieghted scan showed a well-defined submucosal tumor that was highly enhanced (B).
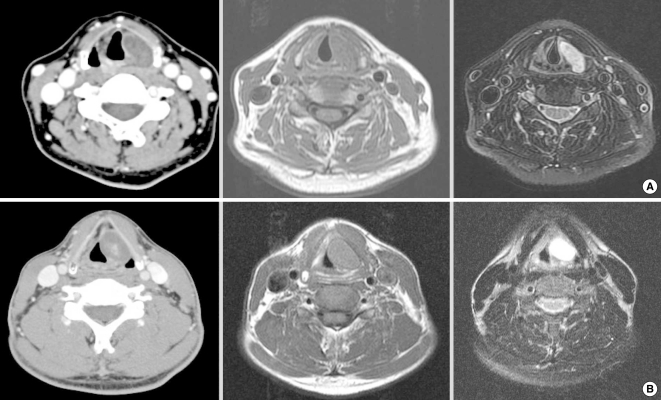
Fig. 3
The intraoperative finding of case 2. The incision was marked on the thyroid cartilage (A). The laryngeal schwannoma was dissected by fingers through the lateral thyroid window (B). The thyroid cartilage was reapproximated (C).
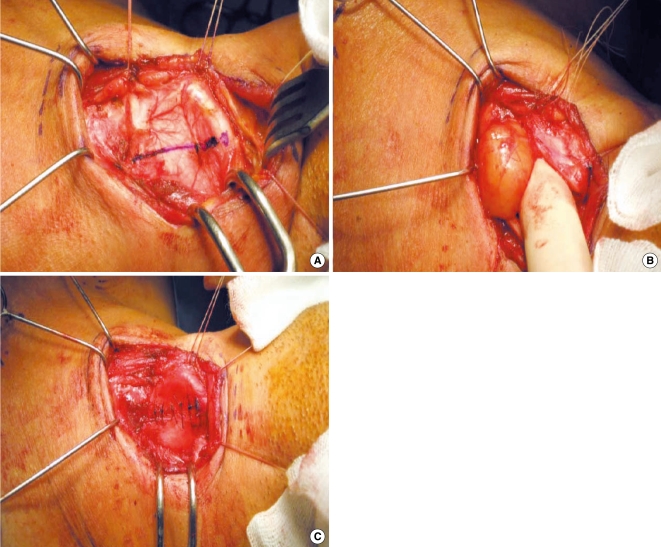
Fig. 4
The histologic findings of the schwannoma in case 2. The mass had a yellowish color and it had a well capsulated sursolid form (A). The schwannoma cells were organized in compact bundles with the nuclei arranged in a palisade manner (Antoni type A) and the cells were partly dispersed with loose reticular fibers (Antoni type B) (B: H&E, ×100; C: H&E, ×400). The schwannoma cells showed immunoreactivity for S-100 protein (D: Immunohistochemistry).




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download