INTRODUCTION
MATERIALS AND METHODS
Preparation of samples
HEI-OC1 cell culture
Semi-quantitative RT-PCR
MTT assay
Hoechst 33258 staining
Confocal immunofluorescence imaging
Western blotting
RESULTS
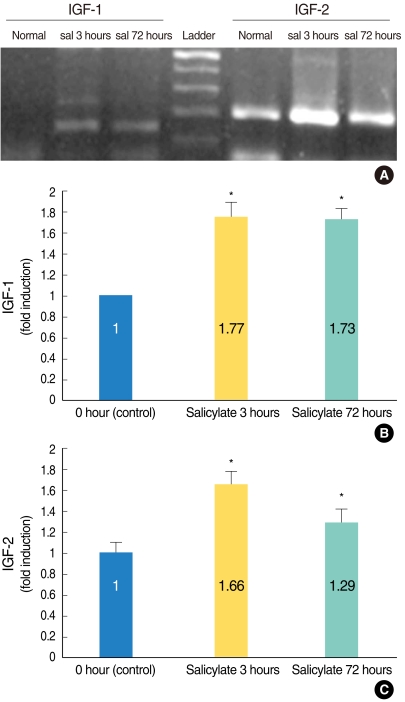 | Fig. 1Semi-quantitative reverse transcription polymerase chain reaction (RT-PCR) analysis confirmed that the insulin-like growth factors (IGFs)' mRNA expressions were significantly enhanced in the salicylate (sal) ototoxicity groups as compared with that of the normal control group (A). The ratio of the expression levels on the image of the RT-PCR and as measured by densitometry was significantly higher in the salicylate group (and especially the salicylate 3 hours group) as compared to that of the normal control group (B, IGF-1; C, IGF-2). *P<0.05 compared with the control group. |
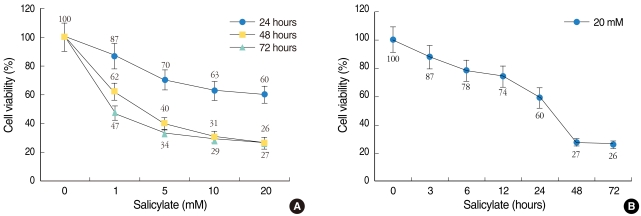 | Fig. 2Cytotoxic effect of salicylate on the HEI-OC1 cell cultures. Cell viability was measured using the MTT assay and this was expressed as a percentage of the untreated control cell samples. Dose-dependent (A) or time-dependent (B) effects of salicylate on the HEI-OC1 cell cultures. The cells treated with 1, 5, 10, or 20 mM salicylate for 24 (●), 48 (■), and 72 (▲) hours (A), and with salicylate (at 20 mM) for 0, 3, 6, 12, 24, 48, and 72 hours (B). The values are the mean±SE of the data. |
 | Fig. 3Hoechst 33258 staining of the salicylate-treated HEI-OC1 cell cultures. The HEI-OC1 cell cultures were treated without salicylate for 24 hours (A), or with salicylate at 10 mM (B) and 20 mM (C) for 24 hours, and they were examined under a light microscope. The nuclei of the normal control cells were shown as round-shaped with homogenous staining (A). However, the 10 mM and 20 mM salicylate treatment induced characteristic apoptotic morphology nuclei with heterogeneous staining, condensation and fragmentation (B, C). |
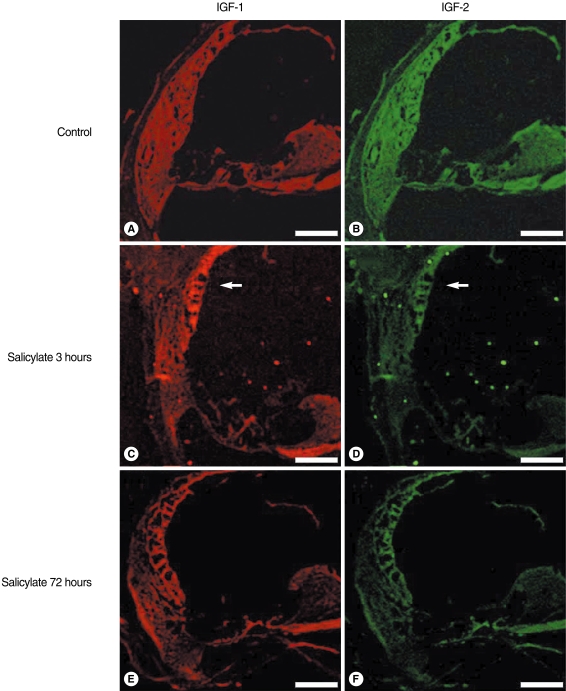 | Fig. 4Confocal immunofluorescence imaging of the insulin-like growth factors (IGFs) in salicylate ototoxicity. The IGFs expressions were non-specific in the normal control group (A, B). IGF-1 and 2 were highly expressed in the stria vascularis (arrow) 3 hours after an injection of sodium salicylate, as compared with that of the normal control group and the salicylate 72 hours group (C, D). Decreased IGF expressions were observed in the salicylate 72 hours group, which is the time of returning to normal hearing (E, F). Scale bars is 100 µm. |
Western blotting
 | Fig. 5Western blot analysis of the insulin-like growth factors (IGFs) in salicylate (sal) ototoxicity. The expressions of IGF-1 and 2 were increased in the sal ototoxicity groups compared with that of the normal control group. The expressions of IGFs on the in-vitro study (control, sal 10 mM and 20 mM) were increased in a dose-dependent manner. The expression of IGFs in the in-vivo study was higher in the sal group (and especially the sal 3 hours group) than that of the normal control. The molecular weight of the IGF-1 and 2 proteins was about 7.6 and 7.4 kDa, respectively. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download