Abstract
Objectives.
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a more severe inflammatory form of CRS that often coexists with obstructive sleep apnea (OSA). However, little is known about the relationship between OSA and the immune profile in patients with CRSwNP. We aimed to investigate the immune profile of patients with CRSwNP according to OSA severity.
Methods.
This study included 63 patients with CRSwNP and nine control subjects. Protein levels of inflammatory mediators were determined using multiplex immunoassays. All patients underwent standard polysomnography.
Results.
In patients with eosinophilic CRSwNP (ECRSwNP), interleukin (IL)-6 and chemokine [C-X-C motif] ligand (CXCL)-1 (type 1 immune-related markers) were upregulated in cases of moderate-to-severe OSA. Additionally, IL-4, IL-13, C-C motif chemokine (CCL)-11, CCL-24 (type 2 immune-related markers), and IL-17A (a type 3 immune-related marker) were present at elevated levels in patients with moderate-to-severe OSA. Although there were no significant differences in type 1, 2, or 3 immune-related markers among patients with non-eosinophilic CRSwNP (NECRSwNP) according to the severity of OSA, transforming growth factor-beta expression was higher in those with moderate-to-severe OSA. Furthermore, in ECRSwNP with moderate-to-severe OSA, associations were detected between serum markers and some upregulated inflammatory markers.
Chronic rhinosinusitis (CRS) is defined as a chronic inflammatory disorder that involves the nasal and paranasal mucosa and lasts for more than 12 weeks [1]. Its major symptoms include nasal obstruction, nasal discharge, facial pain, and reduction or loss of smell. However, in addition to sinonasal symptoms, patients with CRS also suffer from poor sleep quality due to nasal congestion, upper airway obstruction, and inflammatory status. Previous studies demonstrated that patients with CRS commonly experience poor sleep quality and have highly prevalent sleep problems compared to individuals without CRS [2]. A recent study found that incident CRS was associated with impaired sleep quality [3].
Obstructive sleep apnea (OSA) is characterized by repeated cessation of breathing during sleep that is primarily caused by complete or partial airway obstruction [4,5]. These episodes of airway obstruction induce nocturnal hypoxemia, hypercapnia, and sleep fragmentation. OSA can also cause or exacerbate severe major-organ disorders, including cardiovascular disease, metabolic syndrome, and neurocognitive deterioration. Moreover, a meta-analysis revealed higher levels of systemic inflammatory markers in patients with OSA than in controls [6]. Several studies have also reported that OSA significantly influenced the sinonasal local immune response, including nitric oxide production, lipid peroxidation, nuclear factor-κB activation, and neutrophil infiltration [7,8].
CRS is divided into two phenotypes according to the presence of nasal polyps under nasal endoscopy: CRS with nasal polyps (CRSwNP) and CRS without nasal polyps. Generally, CRSwNP is considered to reflect a more severe inflammatory status of the sinonasal mucosa, and it also could cause the development or aggravation of apnea during sleep [9]. Further, as a result of intermittent hypoxia due to breathing cessation during sleep, OSA could affect the immunological response of patients with CRSwNP. However, the effect of OSA on the immune systems of patients with CRSwNP is still poorly understood. Therefore, in the present study, we investigated whether OSA influences the immune profile of patients with CRSwNP.
This study was approved by the Institutional Review Board of Hallym Medical University Chuncheon Sacred Hospital (No. 2018-39), and all participants provided written informed consent for study participation. NP tissues were obtained from patients with CRSwNP during routine functional endoscopic sinus surgery. The diagnosis of CRS was based on the European position paper on rhinosinusitis and nasal polyps guidelines. In this study, we excluded the subjects as follows: (1) less than 18 years of age, (2) history of prior treatment with antibiotics, systemic or topical corticosteroids, or other immune-modulating drugs in the 4 weeks before surgery, and (3) presence of unilateral rhinosinusitis, antrochoanal polyp, allergic fungal sinusitis, cystic fibrosis, or immotile ciliary disease. Control tissue samples were also obtained from patients without any sinonasal diseases during other types of rhinologic surgeries. All samples were homogenized with a mechanical homogenizer at 1,000 rpm for 5 minutes on ice. After homogenization, the suspensions were centrifuged at 3,000 rpm for 10 minutes at 4°C, and the supernatants were separated and stored at –80°C for further analysis of cytokines and other inflammatory mediators. The atopic status of the patients was evaluated using the ImmunoCAP assay (Thermo Scientific, Waltham, MA, USA), which detected immunoglobulin E (IgE) antibodies against six mixtures of common aeroallergens (house dust mites, molds, trees, weeds and grass pollen, and animal dander). Participants were considered atopic if the allergenspecific IgE level was greater than 0.35 KU/L for any one or more of the allergens. A diagnosis of asthma was made by an allergist based on the medical history and lung function analysis, including the methacholine challenge test. In this study, CRSwNP was classified as eosinophilic CRSwNP (ECRSwNP) if eosinophils comprised more than 10% of the inflammatory cell population or as non-eosinophilic CRSwNP (NECRSwNP) if eosinophils comprised less than 10% of the inflammatory cells.
In the present study, we used multiplex cytokine analysis kits (tumor necrosis factor [TNF]-α, interferon [IFN]-γ, interleukin [IL]-4, IL-5, IL-10, IL-13, IL-17A, IL-22, IL-23, chemokine [C-X-C motif] ligand [CXCL]-1, CXCL-8, C-C motif chemokine [CCL]-11, CCL-24, eosinophil cationic protein [ECP], myeloperoxidase [MPO], and transforming growth factor [TGF]-β) obtained from R&D Systems (Cat. No. LMSAHM; Minneapolis, MN, USA). All assays were run in duplicate according to the manufacturer’s protocol. All protein levels in tissue homogenates were normalized to the concentration of total protein (mg/mL). Samples were thawed at room temperature and vortexed to ensure they were well-mixed.
Standard overnight polysomnography (PSG) was performed preoperatively for all patients using a computerized polysomnographic device (Nox-A1; Nox Medical, Reykjavik, Iceland). The sleep stage and respiratory events before and after surgery were scored according to the guidelines of the American Academy of Sleep Medicine. Briefly, apnea was scored using an oronasal thermal sensor when the peak signal excursions dropped by ≥90% of the pre-event baseline, lasted for at least two breaths, and were associated with respiratory effort for the entire period of absent airflow. A respiratory event was scored as a hypopnea if the peak signal excursions dropped by ≥30% of the pre-event baseline using nasal pressure, lasted for at least two breaths, and were associated with ≥3% oxygen desaturation from the preevent baseline or with arousal. The apnea-hypopnea index (AHI) score was the sum of apnea plus the number of hypopneas per hour of sleep. Based on the AHI, the severity of OSA was classified as follows: no OSA, AHI <5 per hour; mild OSA, 5≤ AHI <15 per hour; moderate OSA, 15≤ AHI <30 per hour; severe OSA, AHI ≥30 per hour.
Prior to conducting the study, we performed a power calculation and determined the sample size using G power. R version 3.4.2 software and GraphPad Prism software 7.0 (GraphPad, La Jolla, CA, USA) were used for the statistical analyses. A chi-square test and two-tailed Mann-Whitney U-test were used for unpaired comparisons between two groups. For comparisons among more than two groups, the Kruskal-Wallis test was initially used to identify significant differences. The Bonferroni adjustment was used to adjust the significance level for each comparison. We also used Pearson’s correlation coefficient to measure the statistical relationship. The significance level was set at α=0.05.
We consecutively enrolled CRSwNP patients during the study period, and a total of 63 CRSwNP patients underwent PSG before surgery. Of the patients with CRSwNP, 20 (31.7%) had ECRSwNP and 43 (68.3%) had NECRSwNP. The demographic and clinical characteristics of the participants enrolled in this study are presented in Table 1. Among the enrolled patients with CRSwNP, 59 (93.7%) were diagnosed with OSA and 4 CRSwNP patients were considered as non-OSA. The control subjects also underwent PSG, but none were diagnosed with OSA. The ECRSwNP group consisted of 8 patients with no-to-mild OSA and 12 patients with moderate-to-severe OSA, whereas there were 12 patients with no-to-mild OSA and 31 patients with moderate-to-severe OSA in the NECRSwNP group. The demographic and clinical characteristics according to the severity of OSA are presented in Table 2. A significantly higher body mass index was found in patients with moderate to mild OSA (P=0.025). Table 3 presents the data for sleep quality, such as the sleep-related questionnaire and sleep parameters from PSG.
To investigate patients’ immune profile, we performed enzymelinked immunosorbent assay on sinonasal tissues. The analysis of type 1 inflammation among the three groups showed significant differences in CXCL-1, CXCL-2, and IL-6 expression; however, there were no significant differences in IFN-γ, TNF-α, or MPO expression among the three groups (Fig. 1A). TNF-α expression was also higher in the NECRSwNP group than in the control group. Meanwhile, we detected significantly higher expression of various type 2-related inflammatory markers, including IL-4, IL-5, IL-13, CCL-11, and CCL-24 in patients with ECRSwNP (Fig. 1B). In the analysis of type 3 inflammation, IL-22 expression was significantly higher in the ECRSwNP and NECRSwNP groups than in the control group, whereas there were no significant differences in IL-17A or IL-23 among the three groups (Fig. 1C). TGF-β expression also showed significant downregulation in patients with ECRSwNP and NECRSwNP.
To elucidate the effect of OSA in CRSwNP, we compared the expression of several inflammatory markers in NP tissues between patients with no-to-mild OSA and those with moderate-to-severe OSA. Interestingly, in the patients with ECRSwNP, we found that the expression of several type 1 (CXCL-1 and IL-6), type 2 (IL-4, IL-13, CCL-11, and CCL-24), and type 3 (IL-17A) inflammatory markers were significantly upregulated in patients with moderate-to-severe OSA compared to those with no-to-mild OSA (Fig. 2). In contrast, there were no significant differences between the NECRSwNP patients with no-to-mild OSA and those with moderate-to-severe OSA in the expression of type 1, 2, or 3 inflammatory markers in NP tissues. Moreover, patients with NECRSwNP showed only increased TGF-β expression in NP tissues in cases of moderate-to-severe OSA compared to cases with no-to-mild OSA (Fig. 3).
Next, to elucidate the relationship between the systemic and local immune response in patients with ECRSwNP and moderate-to-severe OSA, we analyzed the correlations of upregulated inflammatory mediators with serum markers, such as eosinophil count and C-reactive protein (CRP) (Fig. 4). CRP was significantly associated with IL-6 (r=0.916, P=0.002) and CXCL-1 (r=0.713, P=0.047), but not IL-17A. No significant association was found between serum eosinophil count and type 2 inflammatory markers; however, we found nearly significant associations between serum eosinophil count and IL-4 (r=0.702, P=0.052) and CCL-11 (r=0.689, P=0.059).
Several previous studies have demonstrated relationships between CRS and OSA. In addition, some studies have shown improvements in sleep quality in CRS patients after endoscopic sinus surgery [10,11]. OSA also may be associated with changes that affect the components and responses of the immune system, as sleep deprivation impairs host defense mechanisms [12]. Moreover, a recent study of the nasal microbiome showed that the severity of OSA was correlated with differences in microbiome diversity and composition in the sinonasal cavity [13]. However, to the best of our knowledge, no studies have examined the effects of OSA on the immune profile of patients with CRSwNP. In this study, we investigated the immune profile of patients with CRSwNP according to the severity of OSA. We found that IL-6 and CXCL-1 (type 1 immune-related markers); IL-4, IL-13, CCL-11, CCL-24 (type 2 immune-related markers); and IL17A (a type 3 immunerelated marker) levels were upregulated in cases of moderate-tosevere OSA in ECRSwNP, but not in NECRSwNP. Therefore, we suggest that OSA may contribute to an increase in immune profile heterogeneity in patients with ECRSwNP.
ECRSwNP is characterized by type 2 immune inflammation with increased eosinophil infiltration, whereas NECRSwNP shows mixed (types 1, 2, and 3) immune inflammation with predominant neutrophilic infiltration [14,15]. In accordance with previous reports, our study revealed that ECRSwNP primarily showed higher type 2 immune inflammation compared to NECRSwNP, regardless of OSA severity. Interestingly, we showed that, in patients with ECRSwNP, type 1-, 2-, and 3-related inflammatory cytokines were upregulated in moderate-to-severe OSA compared to no-to-mild OSA. However, unlike ECRSwNP, patients with NECRSwNP had no significant differences in type 1-, 2-, or 3-related inflammatory cytokine expression according to OSA severity. Additionally, in ECRSwNP patients with moderate-to-severe OSA, CRP levels in serum were significantly associated with nasal neutrophilic inflammation markers, such as IL-6 and CXCL-1, and serum eosinophil count showed a linear relation with nasal eosinophilic inflammation markers, such as IL-4 and CCL-11. Collectively, these findings imply that sleep fragmentation and intermittent hypoxia could contribute to changes of the immune profile in patients with ECRSwNP, but not in those with NECRSwNP.
To date, multiple studies have consistently reported that undiagnosed or inadequately treated OSA adversely affects asthma control, whereas continuous positive airway pressure as a treatment for OSA could attenuate the risk for poor asthma outcomes, partially due to the effects of intermittent hypoxia on airway inflammation and tissue remodeling [16,17]. Several other human and animal studies showed that OSA could shift the immune profile in patients with asthma from traditional eosinophilic or type 2 inflammation to neutrophilic or type 1 inflammation [16,18,19]. Additionally, real-world datasets showed an association between OSA and poor asthma control [20]. These studies showed that OSA may be a contributor to increased neutrophilrelated inflammation in asthma, leading to a more heterogeneous immune profile and poor disease control. Similar to prior studies, our study found an increased type 1 (CXCL-1 and IL-6) and type 3 (IL-17A) immune profile in patients with ECRSwNP with moderate-to-severe OSA. Consistent with our findings, prior studies showed that OSA was associated with increased serum levels of CRP, IL-8, IL-6, and TNF-α [21-23]. Several previous studies demonstrated that OSA may be associated with changes in T-cell activation and proliferation, leading to increased CD4+ and CD8+ cell counts [24,25]. A recent study reported that OSA affected the immune response by increasing the proliferative potential of CD4+ and natural killer cells and decreasing phagocytosis and NADPH oxidase activity in neutrophils [26]. Similar to those results, we found upregulated type 2 immune inflammation in patients with ECRSwNP and moderate-to-severe OSA. Collectively, the increased heterogeneity in immune status may be associated with more severe disease expression, remodeling, and poor response to corticosteroids in patients with ECRSwNP and moderate-to-severe OSA.
Generally, NECRSwNP is regarded as an extrinsic form of CRS, since its inflammation originates from external stimuli such as bacteria and allergens, rather than intrinsic mucosal abnormalities [27]. Thus, NECRSwNP usually shows enhanced epithelial alterations and more localized maxillary involvement than ECRSwNP [28,29]. For these reasons, we assumed that the immune profile of patients with NECRSwNP was relatively strongly affected by localized immune status (such as sinonasal tissue), while systemic immune status (such as in asthma) more extensively affected the immune profile of patients with ECRSwNP. In the present study, we did not find any significant differences in type 1, 2, and 3–related inflammatory markers in the NP tissues of patients with NECRSwNP with no-to-mild and moderate-to-severe OSA. Since OSA should be considered a systemic disease rather than a local abnormality, we do not the change in the immune profile to be definitively established in patients with NECRSwNP. However, TGF-β expression was higher in patients with NECRSwNP with moderate-to-severe OSA than in those with no-to-mild OSA. It has been established that TGF-β plays an important role in remodeling processes involved in CRS and serves as the main switch for different remodeling patterns in CRSwNP [30]. Therefore, OSA severity may partially influence tissue remodeling in patients with NECRSwNP.
As this was a cross-sectional study, it presented some limitations. First, although there was no significant difference in disease severity as shown by the Lund-Mackay computed tomography score for CRSwNP and AHI for OSA, whether OSA severity influences the immune profile of NP tissues or whether CRSwNP severity contributes to the severity of OSA remains unclear. Therefore, a longitudinal study is required to investigate the possibility of changes in NP tissue immunology over time. Nonetheless, we found some clues regarding the effect of systemic immune status on local nasal immunity in ECRSwNP patients with moderate-to-severe OSA. Interestingly, we observed that a higher CRP level, as a systemic immunological factor, was associated with higher expression of nasal neutrophil inflammatory markers, such as IL-6 and CXCL-1, and the association between a higher serum eosinophil count, as a systemic immunological factor, and higher expression of nasal type 2 inflammatory markers, such as IL-4 and CCL-11 was nearly significant. These findings imply that sleep fragmentation and intermittent hypoxia might contribute to changes of the immune profile in CRSwNP patients. Secondly, to prove the reliability of these findings, an external validation study is needed.
Our findings suggest that OSA severity influences the immune profile of NP tissues in patients with ECRSwNP. Specifically, a mixed immune response (types 1, 2, and 3) was upregulated in ECRSwNP, whereas we could not find any significant effect in NECRSwNP according to OSA severity.
▪ We investigated the immune profile of patients with chronic rhinosinusitis with nasal polyps (CRSwNP) according to obstructive sleep apnea (OSA) severity.
▪ In patients with eosinophilic CRSwNP, interleukin-6 and chemokine [C-X-C motif] ligand (CXCL)-1 were upregulated with moderate-to-severe OSA, whereas there were no significant differences in any type 1, 2, or 3 immune-related markers in those with non-eosinophilic CRSwNP.
▪ OSA may increase the heterogeneity of immune profiles in patients with eosinophilic CRSwNP.
ACKNOWLEDGMENTS
This research was supported by a clinical research grant-in-aid from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (NRF-2018R1D1A3B07040-862, and by the Hallym University Research Fund 2019 (HURF-2019-59).
REFERENCES
1. Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012; Mar. 50(1):1–12.

2. Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002; Apr. 6(2):97–111.

3. Bengtsson C, Jonsson L, Holmstrom M, Hellgren J, Franklin K, Gislason T, et al. Incident chronic rhinosinusitis is associated with impaired sleep quality: results of the RHINE study. J Clin Sleep Med. 2019; Jun. 15(6):899–905.

4. Liu SY, Riley RW, Yu MS. Surgical algorithm for obstructive sleep apnea: an update. Clin Exp Otorhinolaryngol. 2020; Aug. 13(3):215–224.

5. Yu MS, Ibrahim B, Riley RW, Liu SY. Maxillomandibular advancement and upper airway stimulation: extrapharyngeal surgery for obstructive sleep apnea. Clin Exp Otorhinolaryngol. 2020; Aug. 13(3):225–33.

6. Nadeem R, Molnar J, Madbouly EM, Nida M, Aggarwal S, Sajid H, et al. Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J Clin Sleep Med. 2013; Oct. 9(10):1003–12.

7. Greenberg H, Ye X, Wilson D, Htoo AK, Hendersen T, Liu SF. Chronic intermittent hypoxia activates nuclear factor-kappaB in cardiovascular tissues in vivo. Biochem Biophys Res Commun. 2006; May. 343(2):591–6.
8. Hamada S, Tatsumi S, Kobayashi Y, Yasuba H. Nasal nitric oxide improved by continuous positive airway pressure therapy for upper airway inflammation in obstructive sleep apnea. Sleep Breath. 2017; May. 21(2):405–10.

9. Alt JA, Smith TL. Chronic rhinosinusitis and sleep: a contemporary review. Int Forum Allergy Rhinol. 2013; Nov. 3(11):941–9.

10. Hui JW, Ong J, Herdegen JJ, Kim H, Codispoti CD, Kalantari V, et al. Risk of obstructive sleep apnea in African American patients with chronic rhinosinusitis. Ann Allergy Asthma Immunol. 2017; Jun. 118(6):685–8.e1.

11. Varendh M, Johannisson A, Hrubos-Strom H, Andersson M. Sleep quality improves with endoscopic sinus surgery in patients with chronic rhinosinusitis and nasal polyposis. Rhinology. 2017; Mar. 55(1):45–52.

12. Dinges DF, Douglas SD, Zaugg L, Campbell DE, McMann JM, Whitehouse WG, et al. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest. 1994; May. 93(5):1930–9.

13. Wu BG, Sulaiman I, Wang J, Shen N, Clemente JC, Li Y, et al. Severe obstructive sleep apnea is associated with alterations in the nasal microbiome and an increase in inflammation. Am J Respir Crit Care Med. 2019; Jan. 199(1):99–109.

14. Wang H, Pan L, Liu Z. Neutrophils as a protagonist and target in chronic rhinosinusitis. Clin Exp Otorhinolaryngol. 2019; Nov. 12(4):337–47.

15. Kim DW. Can neutrophils be a cellular biomarker in Asian chronic rhinosinusitis. Clin Exp Otorhinolaryngol. 2019; Nov. 12(4):325–26.

16. Teodorescu M, Broytman O, Curran-Everett D, Sorkness RL, Crisafi G, Bleecker ER, et al. Obstructive sleep apnea risk, asthma burden, and lower airway inflammation in adults in the Severe Asthma Research Program (SARP) II. J Allergy Clin Immunol Pract. 2015; Jul-Aug. 3(4):566–75.e1.

17. Teodorescu M, Polomis DA, Gangnon RE, Fedie JE, Consens FB, Chervin RD, et al. Asthma control and its relationship with obstructive sleep apnea (OSA) in older adults. Sleep Disord. 2013; 2013:251567.

18. Taille C, Rouvel-Tallec A, Stoica M, Danel C, Dehoux M, Marin-Esteban V, et al. Obstructive sleep apnoea modulates airway inflammation and remodelling in severe asthma. PLoS One. 2016; Mar. 11(3):e0150042.

19. Zhang X, Rui L, Wang M, Lian H, Cai L. Sinomenine attenuates chronic intermittent hypoxia-induced lung injury by inhibiting inflammation and oxidative stress. Med Sci Monit. 2018; Mar. 24:1574–80.

20. Nyenhuis SM, Akkoyun E, Liu L, Schatz M, Casale TB. Real-world assessment of asthma control and severity in children, adolescents, and adults with asthma: relationships to care settings and comorbidities. J Allergy Clin Immunol Pract. 2020; Mar. 8(3):989–96.e1.

21. Alberti A, Sarchielli P, Gallinella E, Floridi A, Floridi A, Mazzotta G, et al. Plasma cytokine levels in patients with obstructive sleep apnea syndrome: a preliminary study. J Sleep Res. 2003; Dec. 12(4):305–11.

22. Bouloukaki I, Papadimitriou V, Sofras F, Mermigkis C, Moniaki V, Siafakas NM, et al. Abnormal cytokine profile in patients with obstructive sleep apnea-hypopnea syndrome and erectile dysfunction. Mediators Inflamm. 2014; 2014:568951.

23. Carpagnano GE, Spanevello A, Sabato R, Depalo A, Palladino GP, Bergantino L, et al. Systemic and airway inflammation in sleep apnea and obesity: the role of ICAM-1 and IL-8. Transl Res. 2010; Jan. 155(1):35–43.

24. Series F, Chakir J, Boivin D. Influence of weight and sleep apnea status on immunologic and structural features of the uvula. Am J Respir Crit Care Med. 2004; Nov. 170(10):1114–9.

25. Kim J, Bhattacharjee R, Dayyat E, Snow AB, Kheirandish-Gozal L, Goldman JL, et al. Increased cellular proliferation and inflammatory cytokines in tonsils derived from children with obstructive sleep apnea. Pediatr Res. 2009; Oct. 66(4):423–8.

26. Said EA, Al-Abri MA, Al-Saidi I, Al-Balushi MS, Al-Busaidi JZ, AlReesi I, et al. Altered blood cytokines, CD4 T cells, NK and neutrophils in patients with obstructive sleep apnea. Immunol Lett. 2017; Oct. 190:272–8.

27. Khalmuratova R, Shin HW. Crosstalk between mucosal inflammation and bone metabolism in chronic rhinosinusitis. Clin Exp Otorhinolaryngol. 2021; Feb. 14(1):43–9.

28. Ishitoya J, Sakuma Y, Tsukuda M. Eosinophilic chronic rhinosinusitis in Japan. Allergol Int. 2010; Sep. 59(3):239–45.

Fig. 1.
Expression of inflammatory mediators in nasal tissues according to the phenotype of chronic rhinosinusitis: (A) type 1 immune-related markers, (B) type 2 immune-related markers, (C) type 3 immune-related markers (results reported as median and interquartile range). ECRSwNP, eosinophilic chronic rhinosinusitis with nasal polyps; NECRSwNP, non-eosinophilic chronic rhinosinusitis with nasal polyps; IFN, interferon; TNF, tumor necrosis factor; MPO, myeloperoxidase; CXCL, chemokine [C-X-C motif] ligand; IL, interleukin; CCL, C-C motif chemokine; ECP, eosinophil cationic protein; TGF, transforming growth factor. *P<0.05, **P<0.01, ***P<0.001.
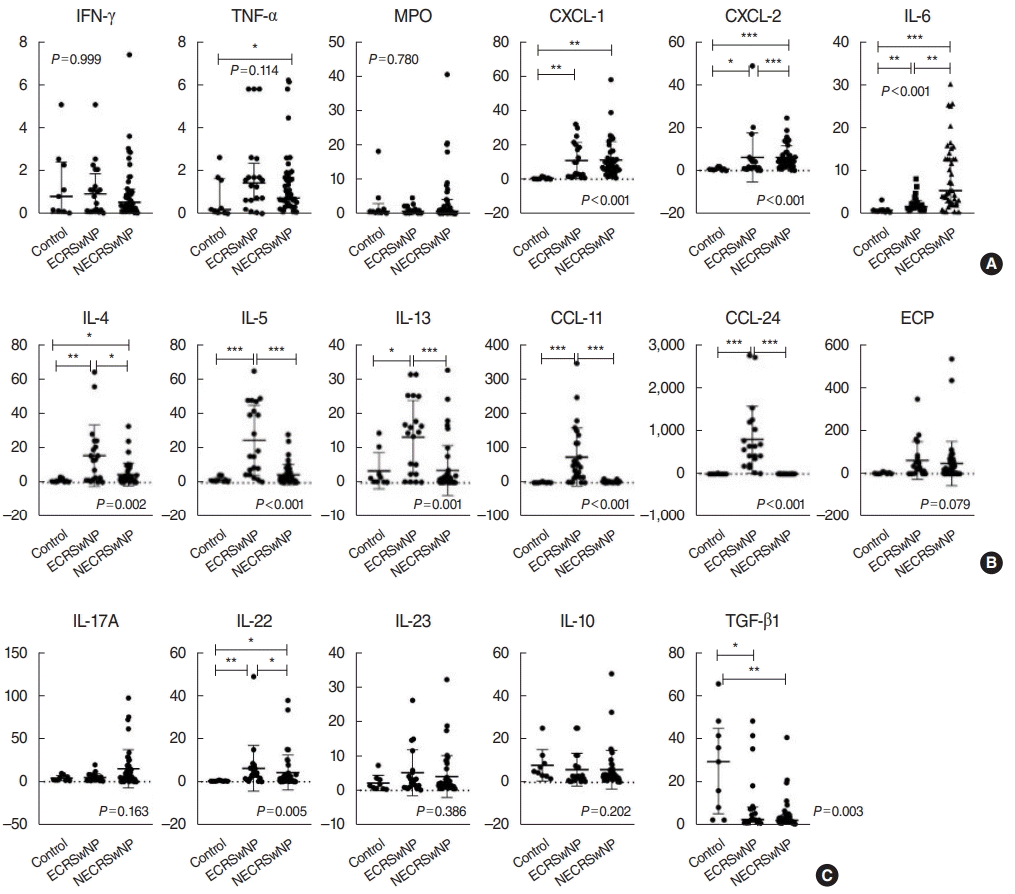
Fig. 2.
Expression of inflammatory mediators in eosinophilic chronic rhinosinusitis with nasal polyps (ECRSwNP) patients according to the severity of OSA: (A) type 1 immune-related markers, (B) type 2 immune-related markers, (C) type 3 immune-related markers (results reported as median and interquartile range). OSA, obstructive sleep apnea; IFN, interferon; TNF, tumor necrosis factor; MPO, myeloperoxidase; CXCL, chemokine [C-X-C motif] ligand; IL, interleukin; CCL, C-C motif chemokine; ECP, eosinophil cationic protein; TGF, transforming growth factor.
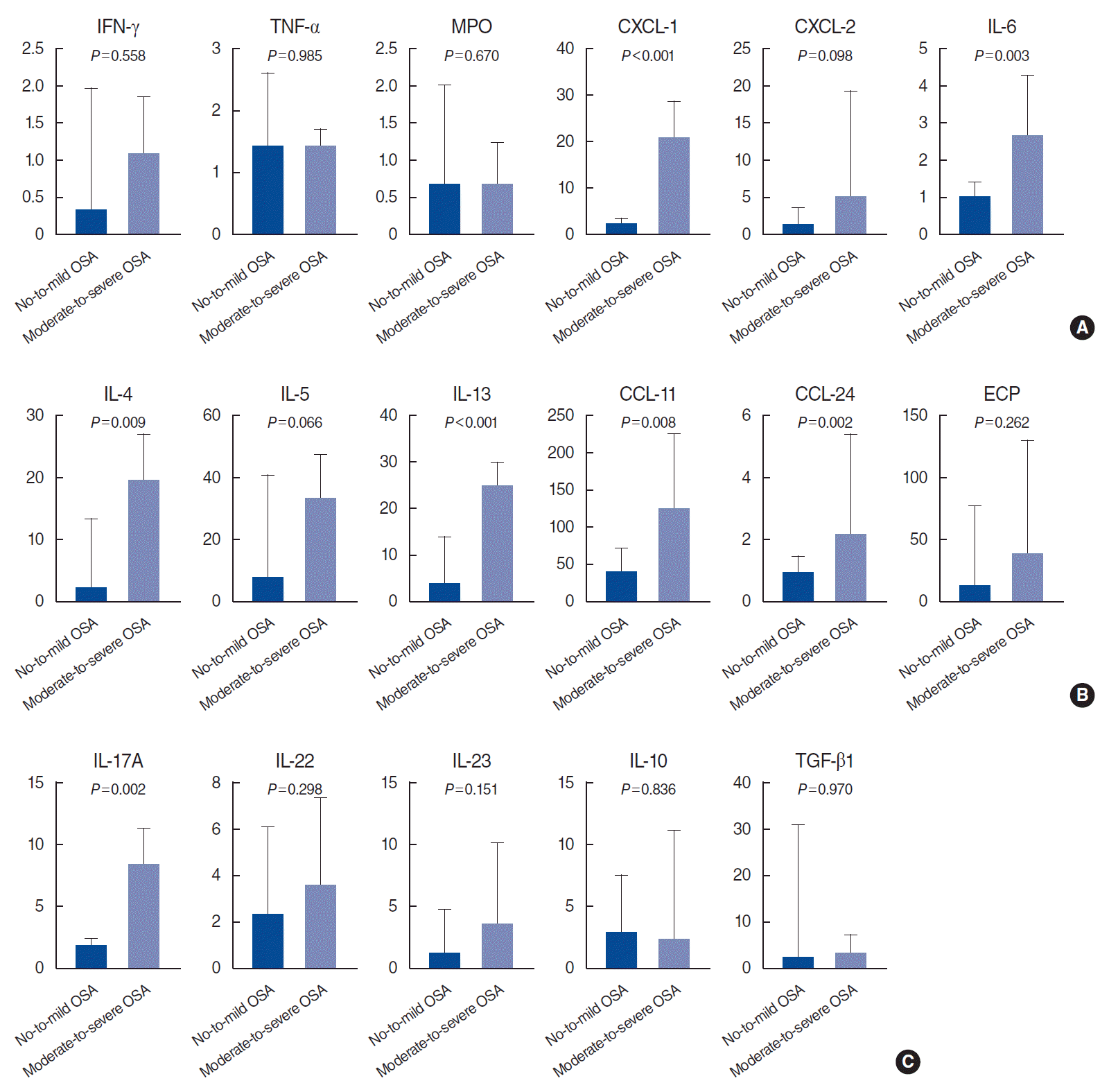
Fig. 3.
Expression of inflammatory mediators in non-eosinophilic chronic rhinosinusitis with nasal polyps (NECRSwNP) patients according to the severity of OSA: (A) type 1 immune-related markers, (B) type 2 immune-related markers, (C) type 3 immune-related markers (results reported as median and interquartile range). OSA, obstructive sleep apnea; IFN, interferon; TNF, tumor necrosis factor; MPO, myeloperoxidase; CXCL, chemokine [C-X-C motif] ligand; IL, interleukin; CCL, C-C motif chemokine; ECP, eosinophil cationic protein; TGF, transforming growth factor.

Fig. 4.
Correlations of upregulated inflammatory mediators and serum markers in eosinophilic chronic rhinosinusitis with nasal polyps (ECRSwNP) patients who had moderate-to-severe obstructive sleep apnea (OSA). (A) Associations of CRP with IL-6, CXCL-1, IL-17A. (B) Associations of serum eosinophil count with IL-4, IL-13, CCL-11, and CCL-24. CRP, C-reactive protein; IL, interleukin; CXCL, chemokine [C-X-C motif] ligand; CCL, C-C motif chemokine.
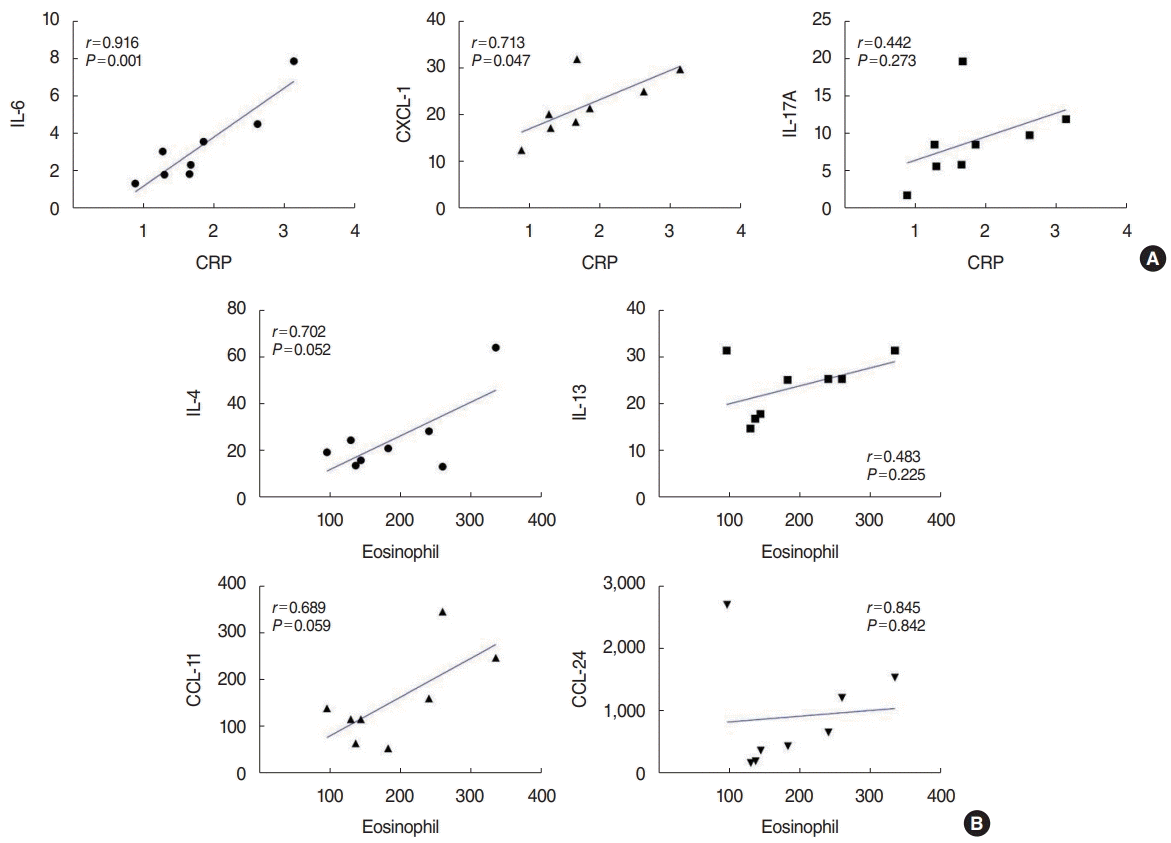
Table 1.
Patient characteristics according to the phenotype of chronic rhinosinusitis with nasal polyps
Table 2.
Characteristics of patients with CRSwNP between no-to-mild and moderate-to-severe OSA
| Variable | No-to-mild OSA (n=24) | Moderate-to-severe OSA (n=39) |
|---|---|---|
| Age (yr) | 52.0±12.9 | 49.2±14.4 |
| Male sex | 19 (79.2) | 32 (82.1) |
| Smoking history | 9 (37.5) | 14 (35.9) |
| Body mass index* (kg/m2) | 23.3±3.5 | 25.8±4.5 |
| Asthma | 4 (16.7) | 5 (12.8) |
| Atopy | 11 (45.8) | 11 (28.2) |
| Aspirin sensitivity | 0 | 0 |
| Serum eosinophil count | 190.7±79.9 | 158.1±185.4 |
| C-reactive protein | 1.0±0.8 | 0.9±0.7 |
| Lund-Mackay CT score | 14.3±4.9 | 15.5±4.9 |
Table 3.
Sleep quality parameters in the study population




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download