1. Lee PJ, Hung PJ, Lee LP. An artificial liver sinusoid with a microfluidic endothelial-like barrier for primary hepatocyte culture. Biotechnol Bioeng. 2007; Aug. 97(5):1340–6.

2. Geraili A, Jafari P, Hassani MS, Araghi BH, Mohammadi MH, Ghafari AM, et al. Controlling differentiation of stem cells for developing personalized organ-on-chip platforms. Adv Healthc Mater. 2018; Jan. 7(2):1700426.

3. Kieninger J, Weltin A, Flamm H, Urban GA. Microsensor systems for cell metabolism: from 2D culture to organ-on-chip. Lab Chip. 2018; May. 18(9):1274–91.
4. Huh D, Torisawa YS, Hamilton GA, Kim HJ, Ingber DE. Microengineered physiological biomimicry: organs-on-chips. Lab Chip. 2012; Jun. 12(12):2156–64.

5. Park H, Lim DJ, Sung M, Lee SH, Na D, Park H. Microengineered platforms for co-cultured mesenchymal stem cells towards vascularized bone tissue engineering. Tissue Eng Regen Med. 2016; Oct. 13(5):465–74.

6. Gurman P, Miranda OR, Clayton K, Rosen Y, Elman NM. Clinical applications of biomedical microdevices for controlled drug delivery. Mayo Clin Proc. 2015; Jan. 90(1):93–108.

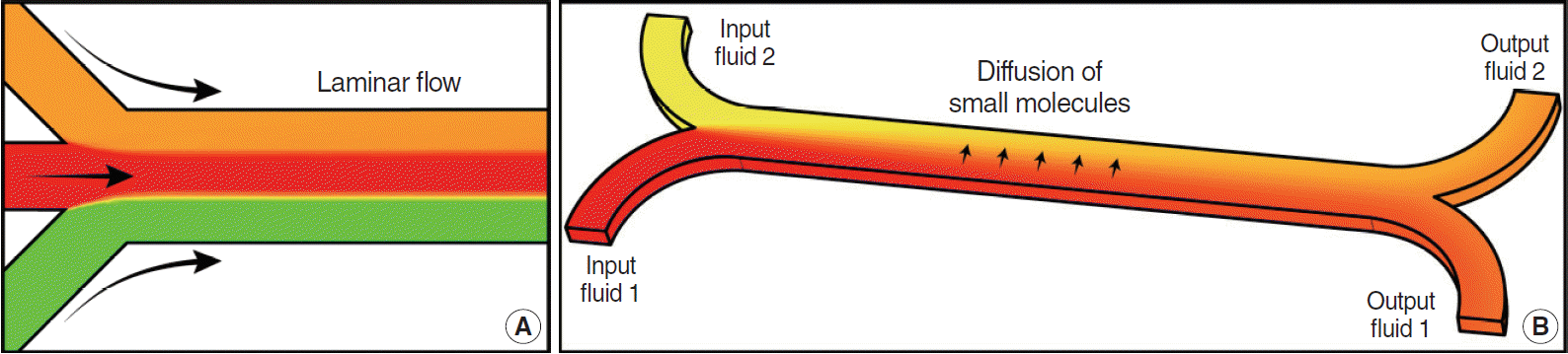
7. Whitesides GM. The origins and the future of microfluidics. Nature. 2006; Jul. 442(7101):368–73.

8. Beebe DJ, Mensing GA, Walker GM. Physics and applications of microfluidics in biology. Annu Rev Biomed Eng. 2002; 4:261–86.

9. Atencia J, Beebe DJ. Controlled microfluidic interfaces. Nature. 2005; Sep. 437(7059):648–55.

10. Li Jeon N, Baskaran H, Dertinger SK, Whitesides GM, Van de Water L, Toner M. Neutrophil chemotaxis in linear and complex gradients of interleukin-8 formed in a microfabricated device. Nat Biotechnol. 2002; Aug. 20(8):826–30.

11. Walker GM, Sai J, Richmond A, Stremler M, Chung CY, Wikswo JP. Effects of flow and diffusion on chemotaxis studies in a microfabricated gradient generator. Lab Chip. 2005; Jun. 5(6):611–8.

12. Agrawal N, Toner M, Irimia D. Neutrophil migration assay from a drop of blood. Lab Chip. 2008; Dec. 8(12):2054–61.

13. Sai J, Rogers M, Hockemeyer K, Wikswo JP, Richmond A. Study of chemotaxis and cell-cell interactions in cancer with microfluidic devices. Methods Enzymol. 2016; 570:19–45.

14. Haque A, Gheibi P, Gao Y, Foster E, Son KJ, You J, et al. Cell biology is different in small volumes: endogenous signals shape phenotype of primary hepatocytes cultured in microfluidic channels. Sci Rep. 2016; Sep. 6:33980.

15. Seemann R, Brinkmann M, Pfohl T, Herminghaus S. Droplet based microfluidics. Rep Prog Phys. 2012; Jan. 75(1):016601.

16. Sackmann EK, Fulton AL, Beebe DJ. The present and future role of microfluidics in biomedical research. Nature. 2014; Mar. 507(7491):181–9.

17. Olanrewaju A, Beaugrand M, Yafia M, Juncker D. Capillary microfluidics in microchannels: from microfluidic networks to capillaric circuits. Lab Chip. 2018; Aug. 18(16):2323–47.

18. Horiuchi T, Hayashi K, Seyama M, Inoue S, Tamechika E. Cooperative suction by vertical capillary array pump for controlling flow profiles of microfluidic sensor chips. Sensors (Basel). 2012; Oct. 12(10):14053–67.

19. Gencturk E, Mutlu S, Ulgen KO. Advances in microfluidic devices made from thermoplastics used in cell biology and analyses. Biomicrofluidics. 2017; Oct. 11(5):051502.

20. Shadpour H, Musyimi H, Chen J, Soper SA. Physiochemical properties of various polymer substrates and their effects on microchip electrophoresis performance. J Chromatogr A. 2006; Apr. 1111(2):238–51.

21. Young EW, Berthier E, Beebe DJ. Assessment of enhanced autofluorescence and impact on cell microscopy for microfabricated thermoplastic devices. Anal Chem. 2013; Jan. 85(1):44–9.

22. Nie J, Gao Q, Wang Y, Zeng J, Zhao H, Sun Y, et al. Vessel-on-a-chip with Hydrogel-based Microfluidics. Small. 2018; Nov. 14(45):e1802368.

23. Zhang X, Li L, Luo C. Gel integration for microfluidic applications. Lab Chip. 2016; May. 16(10):1757–76.

24. Iliescu C, Taylor H, Avram M, Miao J, Franssila S. A practical guide for the fabrication of microfluidic devices using glass and silicon. Biomicrofluidics. 2012; Mar. 6(1):16505–16.

25. Shin DS, Matharu Z, You J, Siltanen C, Vu T, Raghunathan VK, et al. Sensing conductive hydrogels for rapid detection of cytokines in blood. Adv Healthc Mater. 2016; Mar. 5(6):659–64.

26. Yadavali S, Jeong HH, Lee D, Issadore D. Silicon and glass very large scale microfluidic droplet integration for terascale generation of polymer microparticles. Nat Commun. 2018; Mar. 9(1):1222.

27. Markov DA, Lillie EM, Garbett SP, McCawley LJ. Variation in diffusion of gases through PDMS due to plasma surface treatment and storage conditions. Biomed Microdevices. 2014; Feb. 16(1):91–6.

28. Han P, Bartels DM. Temperature dependence of oxygen diffusion in H2O and D2O. J Phys Chem. 1996; Mar. 100(13):5597–602.

29. Unger MA, Chou HP, Thorsen T, Scherer A, Quake SR. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science. 2000; Apr. 288(5463):113–6.

30. Bhattacharya S, Datta A, Berg JM, Gangopadhyay S. Studies on surface wettability of poly(dimethyl) siloxane (PDMS) and glass under oxygen-plasma treatment and correlation with bond strength. J Microelectromech Syst. 2005; Jun. 14(3):590–7.

31. Gonzalez-Suarez AM, Pena-Del Castillo JG, Hernandez-Cruz A, Garcia-Cordero JL. Dynamic generation of concentration- and temporal-dependent chemical signals in an integrated microfluidic device for single-cell analysis. Anal Chem. 2018; Jul. 90(14):8331–6.

32. Park SE, Georgescu A, Huh D. Organoids-on-a-chip. Science. 2019; Jun. 364(6444):960–5.

33. Taylor BJ, Howell A, Martin KA, Manage DP, Gordy W, Campbell SD, et al. A lab-on-chip for malaria diagnosis and surveillance. Malar J. 2014; May. 13:179.

34. Lau D, Walsh JC, Peng W, Shah VB, Turville S, Jacques DA, et al. Fluorescence biosensor for real-time interaction dynamics of host proteins with HIV-1 capsid tubes. ACS Appl Mater Interfaces. 2019; Sep. 11(38):34586–94.

35. Yeh YT, Gulino K, Zhang Y, Sabestien A, Chou TW, Zhou B, et al. A rapid and label-free platform for virus capture and identification from clinical samples. Proc Natl Acad Sci U S A. 2020; Jan. 117(2):895–901.

36. Maerkl SJ. Integration column: microfluidic high-throughput screening. Integr Biol (Camb). 2009; Jan. 1(1):19–29.

37. Volpetti F, Garcia-Cordero J, Maerkl SJ. A microfluidic platform for high-throughput multiplexed protein quantitation. PLoS One. 2015; Feb. 10(2):e0117744.

38. Wu J, Dong M, Rigatto C, Liu Y, Lin F. Lab-on-chip technology for chronic disease diagnosis. NPJ Digit Med. 2018; Apr. 1:7.

39. Kimura Y, Ikeuchi M, Inoue Y, Ikuta K. 3D microdevices that perform sample purification and multiplex qRT-PCR for early cancer detection with confirmation of specific RNAs. Sci Rep. 2018; Nov. 8(1):17480.

40. Kalidoss R, Umapathy S. An overview on the exponential growth of non- invasive diagnosis of diabetes mellitus from exhaled breath by nanostructured metal oxide chemi-resistive gas sensors and μ-preconcentrator. Biomed Microdevices. 2019; Dec. 22(1):2.

41. Choi J, Cho SJ, Kim YT, Shin H. Development of a film-based immunochromatographic microfluidic device for malaria diagnosis. Biomed Microdevices. 2019; Aug. 21(4):86.

42. Qi R, Zhu G, Wang Y, Wu S, Li S, Zhang D, et al. Microfluidic device for the analysis of MDR cancerous cell-derived exosomes’ response to nanotherapy. Biomed Microdevices. 2019; Mar. 21(2):35.

43. Wechsler ME, Stephenson RE, Murphy AC, Oldenkamp HF, Singh A, Peppas NA. Engineered microscale hydrogels for drug delivery, cell therapy, and sequencing. Biomed Microdevices. 2019; Mar. 21(2):31.

44. Fattahi P, Haque A, Son KJ, Guild J, Revzin A. Microfluidic devices, accumulation of endogenous signals and stem cell fate selection. Differentiation. 2020; Mar-Apr. 112:39–46.

45. Mak SY, Li Z, Frere A, Chan TC, Shum HC. Musical interfaces: visualization and reconstruction of music with a microfluidic two-phase flow. Sci Rep. 2014; Oct. 4:6675.

46. Kim YH, Ko KP, Kang IG, Jung JH, Oh DK, Jang TY, et al. Low concentration PM(10) had no effect on nasal symptoms and flow in allergic rhinitis patients. Clin Exp Otorhinolaryngol. 2017; Jun. 10(2):164–7.

47. Khan DA. Allergic rhinitis and asthma: epidemiology and common pathophysiology. Allergy Asthma Proc. 2014; Sep-Oct. 35(5):357–61.

48. Sinurat J, Rengganis I, Rumende CM, Harimurti K. Accuracy of serum-specific IgE test with microfluidic array enzyme-linked immunosorbent assay for diagnosing inhalant allergen sensitization in asthma and/or rhinitis allergy patients in Jakarta, Indonesia. Asia Pac Allergy. 2018; Jan. 8(1):e10.

49. Huang Z, Luo W, Zou X, Liu X, Cai C, Wu Z, et al. Application of biochip microfluidic technology to detect serum allergen-specific immunoglobulin E (sIgE). J Vis Exp. 2019; Apr. (146):e59100.

50. Huang WY, Chou ST, Chen CH, Chou SY, Wu JH, Chen YC, et al. An automatic integrated microfluidic system for allergy microarray chips. Analyst. 2018; May. 143(10):2285–92.

51. Zheng L, Fu Y, Jiang X, Man S, Ran W, Feng M, et al. Microfluidic system for high-throughput immunoglobulin-E analysis from clinical serum samples. Talanta. 2015; Oct. 143:83–9.

52. Bernstein DI, Schwartz G, Bernstein JA. Allergic rhinitis: mechanisms and treatment. Immunol Allergy Clin North Am. 2016; May. 36(2):261–78.
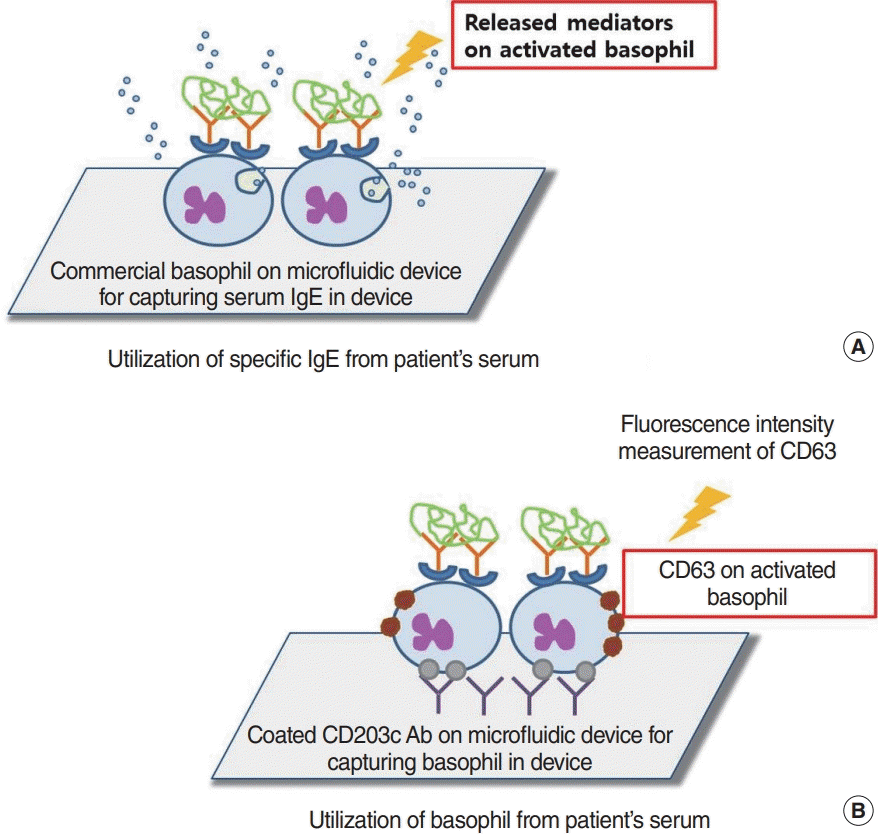
53. Aljadi Z, Kalm F, Nilsson C, Winqvist O, Russom A, Lundahl J, et al. A novel tool for clinical diagnosis of allergy operating a microfluidic immunoaffinity basophil activation test technique. Clin Immunol. 2019; Dec. 209:108268.

54. Hemmings O, Kwok M, McKendry R, Santos AF. Basophil activation test: old and new applications in allergy. Curr Allergy Asthma Rep. 2018; Nov. 18(12):77.

55. Czarnobilska EM, Bulanda M, Spiewak R. The usefulness of the basophil activation test in monitoring specific immunotherapy with house dust mite allergens. Postepy Dermatol Alergol. 2018; Feb. 35(1):93–8.

56. Nopp A, Johansson SG, Ankerst J, Bylin G, Cardell LO, Gronneberg R, et al. Basophil allergen threshold sensitivity: a useful approach to anti-IgE treatment efficacy evaluation. Allergy. 2006; Mar. 61(3):298–302.

57. Kwok HC, Lau PM, Wu SY, Ho HP, Gao M, Kwan YW, et al. Allergy testing and drug screening on an ITO-coated lab-on-a-disc. Micromachines (Basel). 2016; Feb. 7(3):38.

58. Chen QL, Cheung KL, Kong SK, Zhou JQ, Kwan YW, Wong CK, et al. An integrated lab-on-a-disc for automated cell-based allergen screening bioassays. Talanta. 2012; Aug. 97:48–54.

59. Aljadi Z, Kalm F, Ramachandraiah H, Nopp A, Lundahl J, Russom A. Microfluidic immunoaffinity basophil activation test for point-of-care allergy diagnosis. J Appl Lab Med. 2019; Sep. 4(2):152–63.

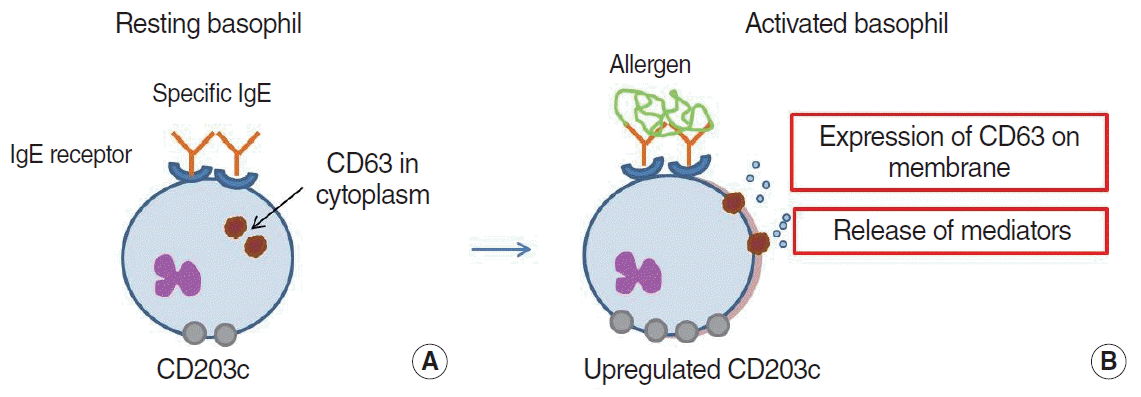
60. Knol EF, Mul FP, Jansen H, Calafat J, Roos D. Monitoring human basophil activation via CD63 monoclonal antibody 435. J Allergy Clin Immunol. 1991; Sep. 88(3 Pt 1):328–38.

61. Yan Y, Gordon WM, Wang DY. Nasal epithelial repair and remodeling in physical injury, infection, and inflammatory diseases. Curr Opin Otolaryngol Head Neck Surg. 2013; Jun. 21(3):263–70.

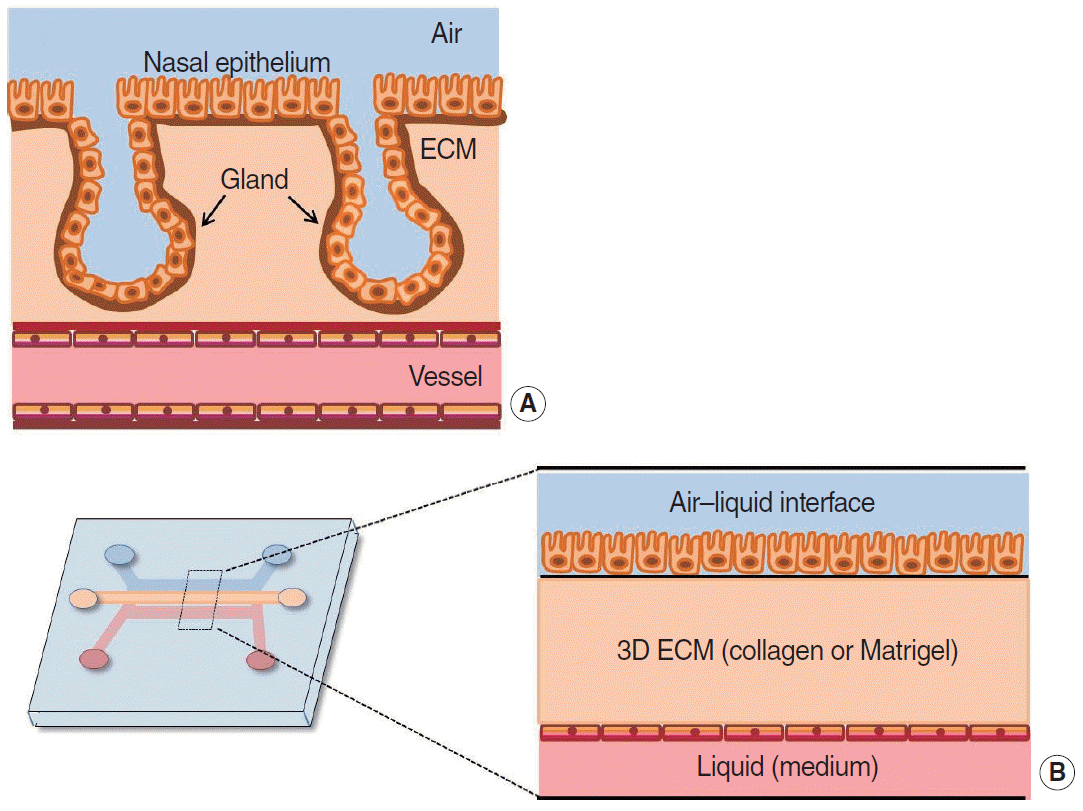
62. Na K, Lee M, Shin HW, Chung S. In vitro nasal mucosa gland-like structure formation on a chip. Lab Chip. 2017; May. 17(9):1578–84.

63. Yu F, Zhao X, Li C, Li Y, Yan Y, Shi L, et al. Airway stem cells: review of potential impact on understanding of upper airway diseases. Laryngoscope. 2012; Jul. 122(7):1463–9.
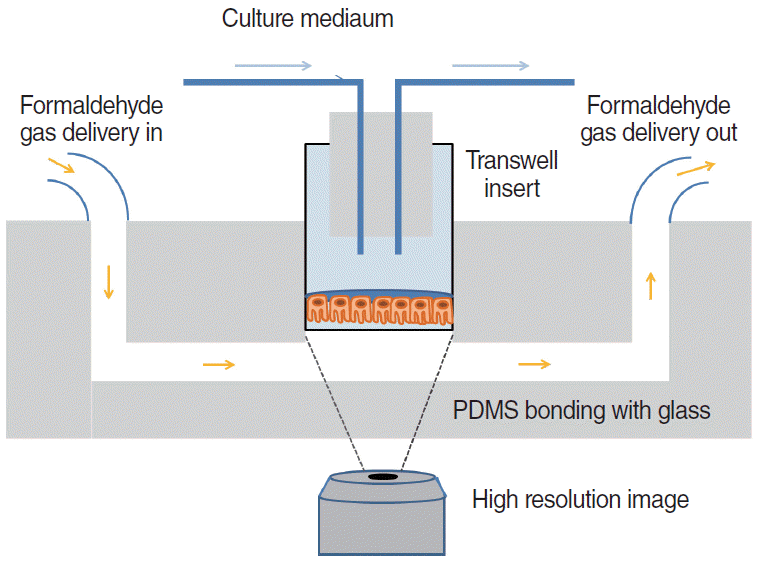
64. Wang W, Yan Y, Li CW, Xia HM, Chao SS, Wang de Y, et al. Live human nasal epithelial cells (hNECs) on chip for in vitro testing of gaseous formaldehyde toxicity via airway delivery. Lab Chip. 2014; Feb. 14(4):677–80.

65. Ayoob AM, Borenstein JT. The role of intracochlear drug delivery devices in the management of inner ear disease. Expert Opin Drug Deliv. 2015; Mar. 12(3):465–79.

66. Shi X. Pathophysiology of the cochlear intrastrial fluid-blood barrier (review). Hear Res. 2016; Aug. 338:52–63.

67. Tandon V, Kang WS, Spencer AJ, Kim ES, Pararas EE, McKenna MJ, et al. Microfabricated infuse-withdraw micropump component for an integrated inner-ear drug-delivery platform. Biomed Microdevices. 2015; Apr. 17(2):37.

68. McCall AA, Swan EE, Borenstein JT, Sewell WF, Kujawa SG, McKenna MJ. Drug delivery for treatment of inner ear disease: current state of knowledge. Ear Hear. 2010; Apr. 31(2):156–65.

69. Brown JN, Miller JM, Altschuler RA, Nuttall AL. Osmotic pump implant for chronic infusion of drugs into the inner ear. Hear Res. 1993; Nov. 70(2):167–72.

70. Chen Z, Kujawa SG, McKenna MJ, Fiering JO, Mescher MJ, Borenstein JT, et al. Inner ear drug delivery via a reciprocating perfusion system in the guinea pig. J Control Release. 2005; Dec. 110(1):1–19.

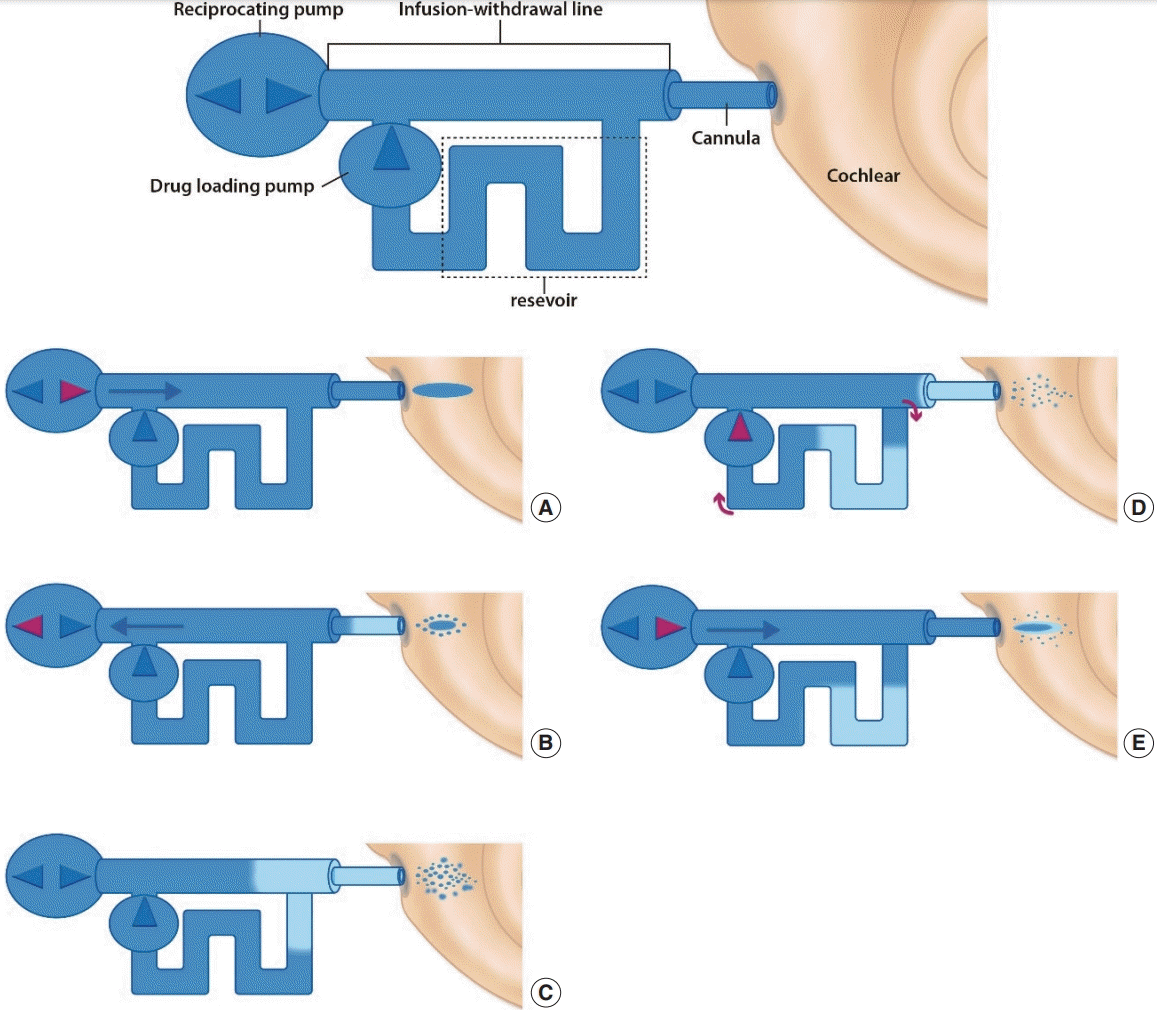
71. Tandon V, Kang WS, Robbins TA, Spencer AJ, Kim ES, McKenna MJ, et al. Microfabricated reciprocating micropump for intracochlear drug delivery with integrated drug/fluid storage and electronically controlled dosing. Lab Chip. 2016; Mar. 16(5):829–46.

72. Sewell WF, Borenstein JT, Chen Z, Fiering J, Handzel O, Holmboe M, et al. Development of a microfluidics-based intracochlear drug delivery device. Audiol Neurootol. 2009; 14(6):411–22.

73. Jahn K. The Aging vestibular system: dizziness and imbalance in the elderly. Adv Otorhinolaryngol. 2019; 82:143–9.

74. Andreou CM, Pahitas Y, Georgiou J. Bio-inspired micro-fluidic angular-rate sensor for vestibular prostheses. Sensors (Basel). 2014; Jul. 14(7):13173–85.
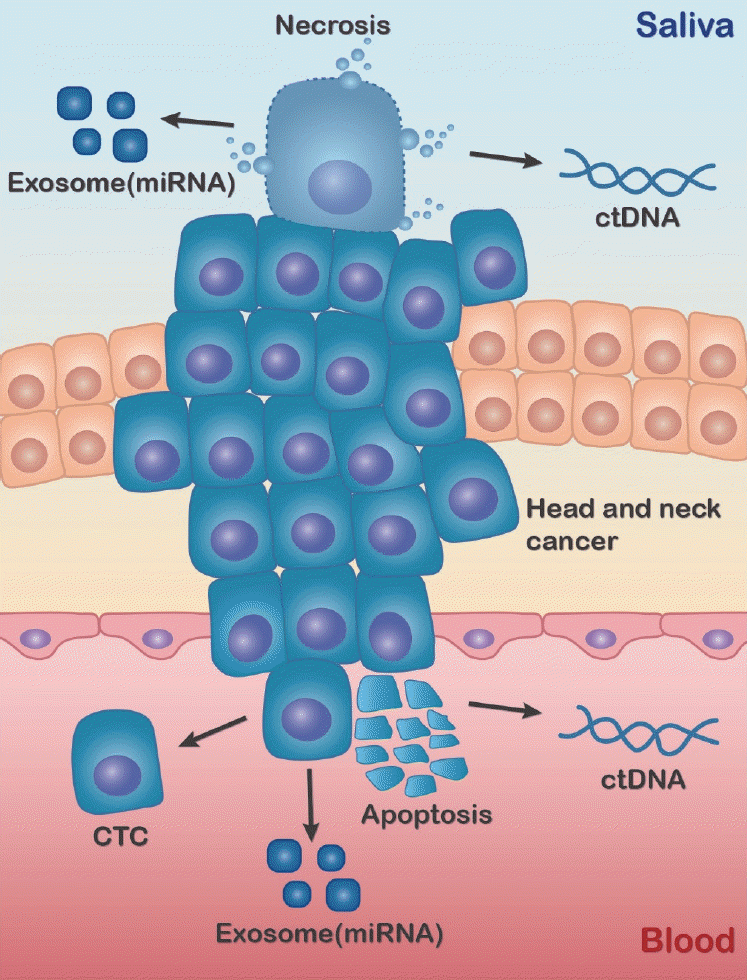
75. Nonaka T, Wong DT. Liquid biopsy in head and neck cancer: promises and challenges. J Dent Res. 2018; Jun. 97(6):701–8.

76. Denaro N, Merlano MC. Immunotherapy in head and neck squamous cell cancer. Clin Exp Otorhinolaryngol. 2018; Dec. 11(4):217–23.

77. Payne K, Brooks J, Spruce R, Batis N, Taylor G, Nankivell P, et al. Circulating tumour cell biomarkers in head and neck cancer: current progress and future prospects. Cancers (Basel). 2019; Aug. 11(8):1115.

78. Wang Z, Li F, Rufo J, Chen C, Yang S, Li L, et al. Acoustofluidic salivary exosome isolation: a liquid biopsy compatible approach for human papillomavirus-associated oropharyngeal cancer detection. J Mol Diagn. 2020; Jan. 22(1):50–9.
79. Zhou Q, Rahimian A, Son K, Shin DS, Patel T, Revzin A. Development of an aptasensor for electrochemical detection of exosomes. Methods. 2016; Mar. 97:88–93.

80. Contreras-Naranjo JC, Wu HJ, Ugaz VM. Microfluidics for exosome isolation and analysis: enabling liquid biopsy for personalized medicine. Lab Chip. 2017; Oct. 17(21):3558–77.

81. Poulet G, Massias J, Taly V. Liquid biopsy: general concepts. Acta Cytol. 2019; 63(6):449–55.

82. Paoletti C, Hayes DF. Circulating tumor cells. Adv Exp Med Biol. 2016; 882:235–58.

83. Garcia SA, Weitz J, Scholch S. Circulating tumor cells. Methods Mol Biol. 2018; 1692:213–9.
84. Cho H, Kim J, Song H, Sohn KY, Jeon M, Han KH. Microfluidic technologies for circulating tumor cell isolation. Analyst. 2018; Jun. 143(13):2936–70.

85. Lin M, Chen JF, Lu YT, Zhang Y, Song J, Hou S, et al. Nanostructure embedded microchips for detection, isolation, and characterization of circulating tumor cells. Acc Chem Res. 2014; Oct. 47(10):2941–50.

86. Gheibi P, Zeng S, Son KJ, Vu T, Ma AH, Dall’Era MA, et al. Microchamber cultures of bladder cancer: a platform for characterizing drug responsiveness and resistance in PDX and primary cancer cells. Sci Rep. 2017; Sep. 7(1):12277.

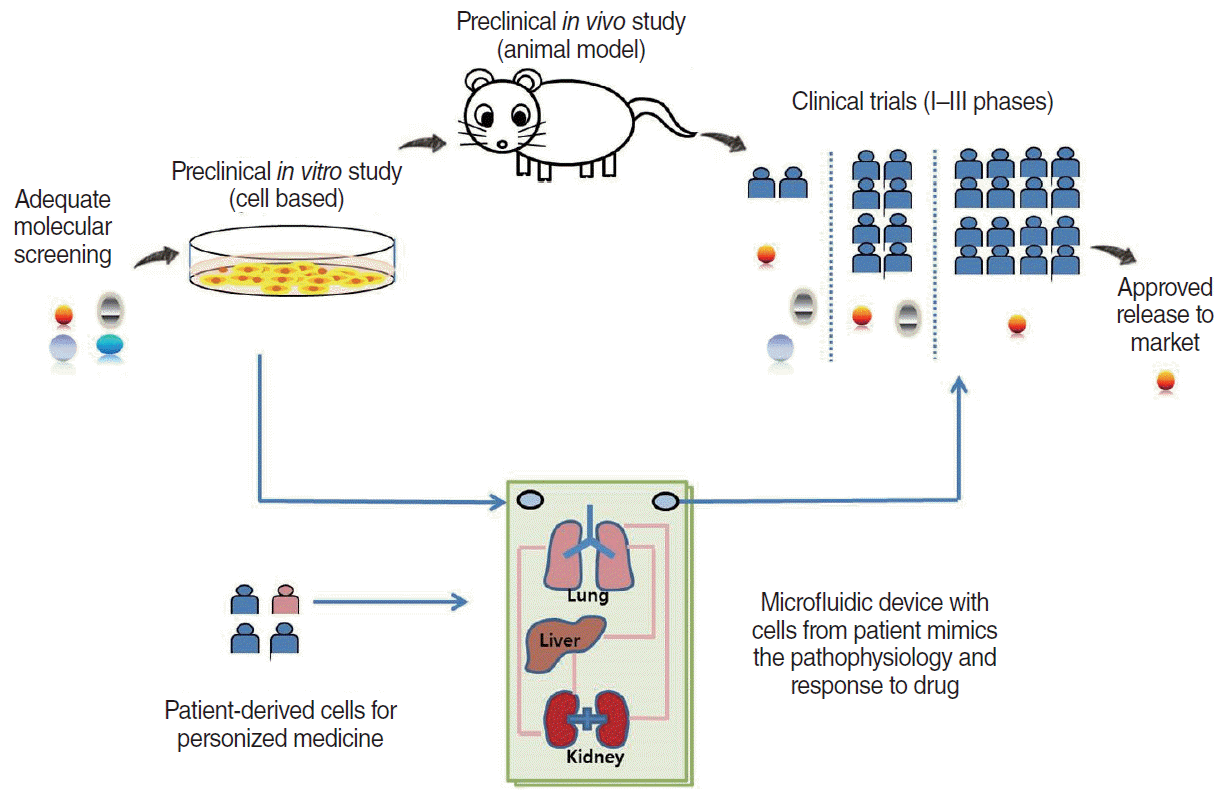
87. Sontheimer-Phelps A, Hassell BA, Ingber DE. Modelling cancer in microfluidic human organs-on-chips. Nat Rev Cancer. 2019; Feb. 19(2):65–81.

88. Zhang H, Zhu Y, Shen Y. Microfluidics for cancer nanomedicine: from fabrication to evaluation. Small. 2018; Jul. 14(28):e1800360.

89. Cheah R, Srivastava R, Stafford ND, Beavis AW, Green V, Greenman J. Measuring the response of human head and neck squamous cell carcinoma to irradiation in a microfluidic model allowing customized therapy. Int J Oncol. 2017; Oct. 51(4):1227–38.

90. Al-Samadi A, Poor B, Tuomainen K, Liu V, Hyytiainen A, Suleymanova I, et al. In vitro humanized 3D microfluidic chip for testing personalized immunotherapeutics for head and neck cancer patients. Exp Cell Res. 2019; Oct. 383(2):111508.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download