Abstract
Objectives
The objectives of this study were to identify the clinical features and chest computed tomography (CT) findings of coronavirus disease 2019 (COVID-19) patients and to compare the characteristics of patients diagnosed in Wuhan and in other areas of China by integrating the findings reported in previous studies.
Methods
We conducted a proportion meta-analysis to integrate the results of previous studies identified in online databases, and subsequently compared the overlapping of 95% confidence intervals (CIs) between locations of diagnosis. The heterogeneity of the results of the included studies was also demonstrated.
Results
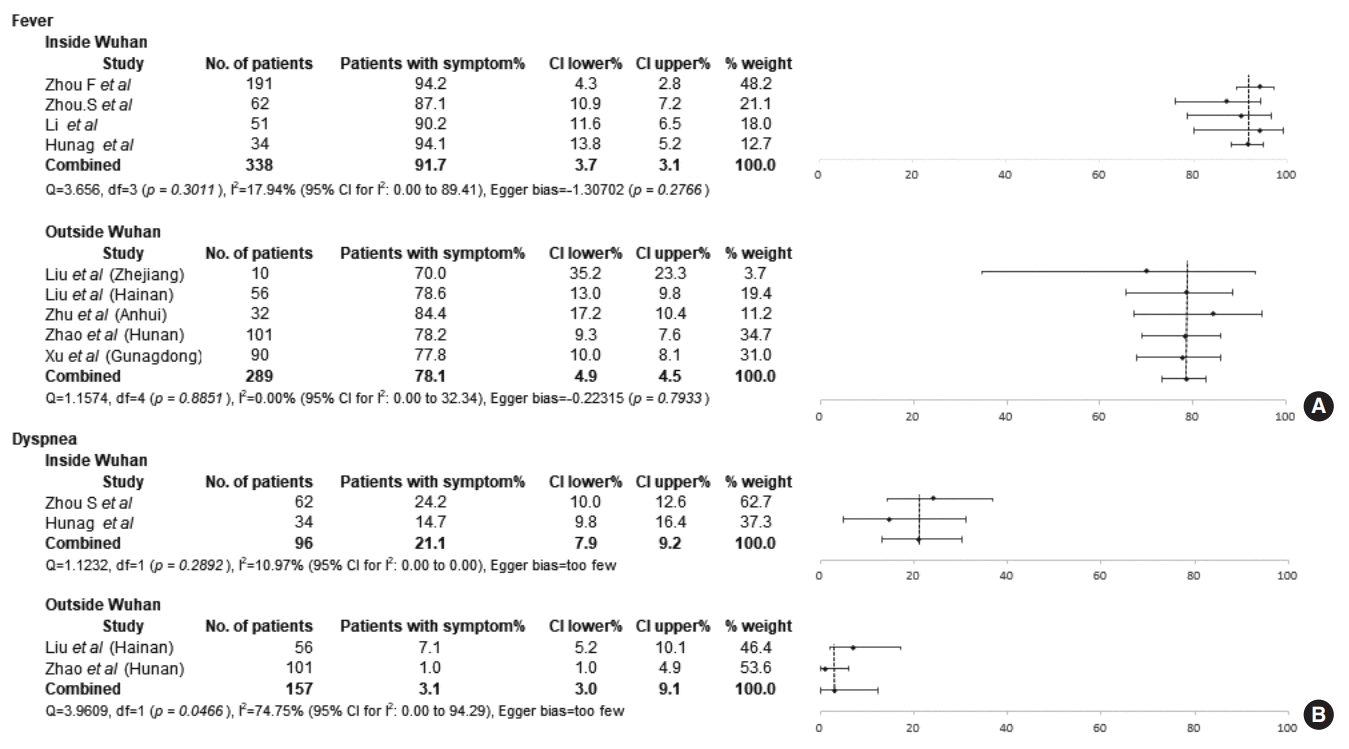
Nine studies with level IV evidence were considered to be eligible for the meta-analysis, and a comparative analysis was only possible between patients diagnosed in Wuhan and outside of Wuhan in China. Fever (84.8%; 95% CI, 78.5% to 90.1%) was identified as the most common clinical manifestation in all COVID-19 patients, and signs of respiratory infection were also frequently present in these patients. When comparing the clinical features according to the location of diagnosis, fever and dyspnea were less frequent in patients diagnosed outside of Wuhan (fever: 78.1%; 95% CI, 73.2% to 82.7%; dyspnea: 3.80%; 95% CI, 0.13% to 12.22%) than in patients diagnosed in Wuhan (fever: 91.7%; 95% CI, 88.0% to 94.8%; dyspnea: 21.1%; 95% CI, 13.2% to 30.3%). The chest CT findings exhibited no significant differences between the groups.
Conclusion
Fever was found to be the most common symptom in COVID-19, and respiratory infection signs were also commonly present. Fever and dyspnea were less frequently observed in the patients diagnosed outside of Wuhan, which should be considered in COVID-19 screening programs. These results may be attributable to the earlier diagnosis of the disease and the younger age of patients outside of Wuhan although further analysis is needed. The role of chest CT in COVID-19 diagnosis is inconclusive based on this study.
During the short period of approximately 3 months since coronavirus disease 2019 (COVID-19) was first reported in the city of Wuhan, Hubei Province, China at the end of December 2019, this disease became a pandemic, with large-scale outbreaks in China, South Korea, Iran, and Europe [1-4]. Although the World Health Organization declared COVID-19 to be a public health emergency of international concern on January 30, 2020 [5], the number of patients diagnosed with COVID-19 in Europe, the United States, and South America exploded in March 2020 [6,7]. The reproductive number of COVID-19 estimated from previously reported studies, representing the average number of new patients infected by a patient in a naïve population, is over 3, which is considerably higher than those of severe acute respiratory syndrome and Middle East respiratory syndrome (MERS) [8,9].
COVID-19 is mainly diagnosed by using real-time reverse transcription-polymerase chain reaction (rRT-PCR) to detect severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA in samples obtained through nasopharyngeal or oropharyngeal swabs [10-13]. Although rRT-PCR for COVID-19 was initially developed within 2 weeks after the emergence of the disease, this test cannot be performed on all suspected patients because COVID-19 has rapidly spread beyond the capacity for testing [14]. Thus, proper screening for the disease and isolation of suspected patients performed by the physicians and healthcare providers who first come into contact with them is the most important step in controlling this highly infectious disease, meaning that awareness and knowledge of the clinical features of COVID-19 are necessary for physicians and health-care providers [15]. The most common symptom in patients diagnosed with COVID-19 is known to be fever, which is frequently accompanied by coughing or difficulty in breathing [16,17]. However, some COVID-19 patients demonstrate no symptoms or complain of only gastrointestinal (GI) symptoms; furthermore, the clinical features of COVID-19 patients diagnosed outside of Wuhan, China differed from those of patients diagnosed in Wuhan [18]. Therefore, additional diagnostic clues for COVID-19 have been investigated; promisingly, chest computed tomography (CT) has recently been reported to detect lung changes caused by COVID19 before the occurrence of symptoms [19,20].
Within only 2 months since the first report of COVID-19, hundreds of reports related to COVID-19 have been published in reputable scientific journals. At this point, it is impossible to conduct a randomized clinical trial or a well-designed prospective study on the diagnosis and treatment of COVID-19, as only a short time has passed since this new disease emerged. Proportion meta-analysis is a statistical technique that provides integrated information in the absence of studies with a high level of evidence [21-24]. It should be kept in mind that this technique provides a relatively low level of evidence compared to other types of meta-analysis, as it generates results from studies with low levels of evidence, such as case series or observational studies [23]. However, this technique is expected to provide more integrated information about COVID-19 for health-care providers than is provided by extant studies in isolation.
Since the first report in Wuhan, the medical environment surrounding COVID-19 has undergone many changes, including increased awareness of the disease and the dissemination of diagnostic kits. Thus, it is expected that there will be changes in the clinical features of patients diagnosed with COVID-19, and identifying such differences will help health-care providers better understand and diagnose this disease. The objectives of this study were to identify the clinical features and chest CT findings of COVID-19 patients and to compare the characteristics of patients diagnosed in Wuhan and in other areas by integrating the findings reported in previous studies.
This study was conducted under the recommendations of Reporting Items for Systematic Reviews and Meta-Analyses. This study was performed by two teams for rapid review, one team was in charge of the survey and collection of included studies, and the other team was in charge of quality assessment of the included studies. Each team consisted of two main reviewers and a supervisor who adjusted the disagreements of the review results. For rapid review considering the urgency of a target disease for analysis, all types of the article what identified in the online databases were considered as candidates for inclusion in the study.
On March 17, 2020, two reviewers (YSL and WJ) searched the online databases of the U.S. National Library of Medicine (Medline) and Excerpta Medica (Embase) for identifying reports published in English that used the terms: COVID-19; SARS-Cov-2; and Novel coronavirus 2019. The titles, abstracts, and authors’ information were saved into excel files, and later screened by two reviewers. All type of reports in the English language that contained the descriptions of clinical features and CT findings except for the review articles were included in a data-set for detailed review, and two reviewers included only studies with data on four or more patients (case-series, cohort, or observational study) into the data-set to provide a higher level of evidence [25]. Published editorials and letters to editors were also considered as candidates for the study to a maximum extent possible. In the review process (the assessments of study eligibility), to minimize the risk of duplicating data in this analysis [26], only the largest study was included if the duration of patient inclusion overlapped among reports from the same hospital. If data were reported from the same hospital without information about the date of patient inclusion, this study was also eventually excluded. All data were collected using identical forms to minimize the variability of reviewers, and disagreements of in the review results were adjusted by the third reviewer (JL).
The extraction of data from included studies was independently conducted by two reviewers. Name of the first author, the origin of reports including hospital and province, the initial date of patient inclusion, and the number of patients included were recorded in the Microsoft Excel database. Demographic data, symptoms of patients, and chest CT findings were extracted. The results were categorized into the regions where COVID-19 patients were diagnosed as follows: Wuhan, outside of Wuhan in China, and other country groups. Quality assessments of included studies were independently performed by two reviewers (professors of college of medicine, CGC and SWP), who did not participate in the data review process, using a quality assessment tool for case-series study from the National Institutes of Health, which consisted of nine questions (Supplementary Table 1) [27]. The disagreements in the quality assessment were also discussed by a third reviewer (JL). The results of the quality assessment were represented in a fair, good, and poor grouping to assist in the understanding of this study.
Proportion meta-analysis was conducted to determine the clinical features and chest CT findings of COVID-19 patients; subsequently, clinical and chest CT characteristics of patients were compared after the areas diagnosed with COVID-19 were classified as Wuhan, outside of Wuhan in China, and other countries. We used a random-effect model provided by MedCalc statistical software due to the presence of uncontrolled variables of included studies. All results were represented as a forest plot with the horizontal bar corresponding to the 95% confidence interval (CI) of the effect estimates, and overlap of 95% CI was defined as the absence of difference among the groups in the comparative analysis [28]. I2 test and Cochran Q were used for evaluating heterogeneities of the results belonging to analysis. I2 lied between 0% to 100% and additional descriptions were made if they complied with the criteria described as follows: low (<40%), moderate (30%–60%), and considerable (75%–100%), retrospectively [29]. This parameter stated the percentage of variability in effect estimates calculated from heterogeneity. Cochran Q test is the parameter computed by summing squared deviations of the estimate of each study, and the parameter of <0.10 determined the heterogeneity [30]. Although we described these two parameters for measuring heterogeneity, I2 which is not affected by the number of included studies for meta-analysis was considered as a more appropriate parameter for the evaluation of heterogeneity in this study [30]. The publication bias among included studies was determined when the P-value was <0.05 on Egger’s regression test [31]. Forest plot calculated by MedCalc was redrawn using Microsoft Excel program (Microsoft, Redmond, WA, USA) for easy interpretation of figure [32]. Chi-square test was used to compare the compositions of gender among groups.
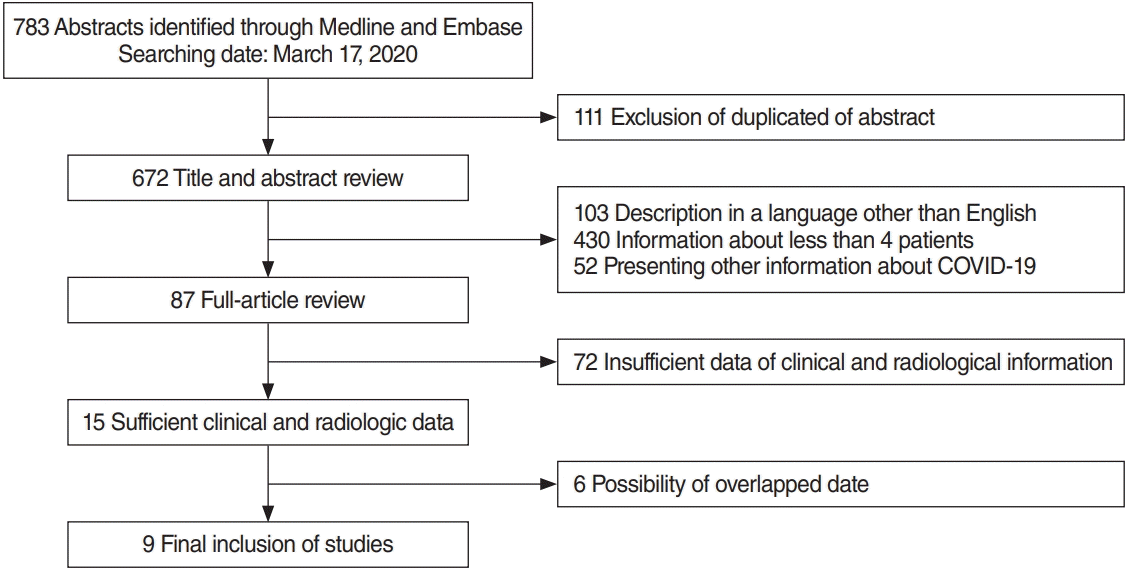
In total, 783 abstracts and titles were identified and screened, and 102 abstracts were excluded in this step as they were not written in English. Additionally, abstracts presenting clinical features and chest CT findings from four or fewer patients were excluded. Eighty-seven reports were completely reviewed, and studies that were likely to present duplicate patient data were also excluded. Finally, nine studies (four studies reporting findings from Wuhan and five studies reporting findings from outside of Wuhan in China) with level IV evidence (case series, observational studies, or cohort studies) were eligible for the meta-analysis (Fig. 1) [33-41]. As only one study each from South Korea and Europe described the clinical features and chest CT findings of patients from outside China, a comparative analysis was only possible between patients diagnosed in Wuhan and outside of Wuhan in China. Information about 627 patients (male, 345; female, 282) diagnosed with COVID-19 was obtained from nine studies (Table 1). However, it was not possible to consistently present the mean or median age of the included patients because five studies reported age as a median value and four studies reported age as a mean value. There was no significant difference in the sex ratio between the two groups (P=0.157) upon chisquare analysis. Among the clinical features described in the included studies, fever, cough, sputum, dyspnea, myalgia, fatigue, and GI symptoms were available for meta-analysis and comparisons (Table 2). Comparisons of chest CT findings were possible for pathologic findings (ground-glass opacity [GGO] and consolidation) and the distribution of abnormal findings (single lobe, multiple lobes, and both lungs) (Table 3). Laboratory findings (leukocytosis, leukopenia, and decreased lymphocyte count) could also be analyzed, as demonstrated in Supplementary Table 2.
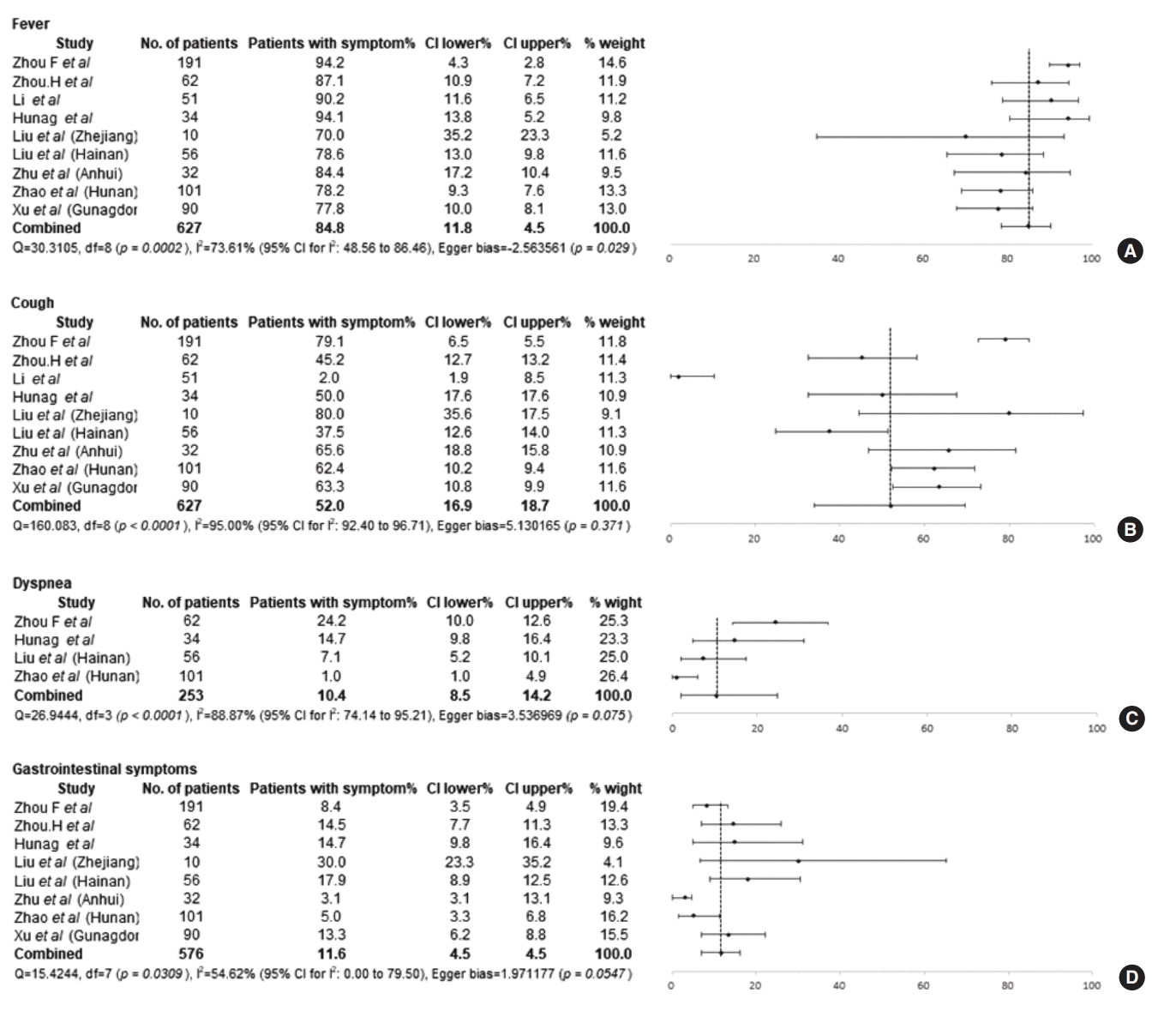
COVID-19 was diagnosed despite the absence of symptoms in 4.1% of the patients (9/242) from outside of Wuhan (Table 2). Fever (84.8%; 95% CI, 78.5% to 90.1%; I2=73.6%) was the most common symptom of COVID-19, followed by cough (52.0%; 95% CI, 34.1% to 69.7%; I2=95.0%) (Fig. 2A and B). Sputum and dyspnea were only present in 21.3% (95% CI, 17.2% to 25.7%; I2=0%) and 10.4% (95% CI, 2.0% to 24.5%; I2=88.87%) of patients, respectively (Fig. 2C). Myalgia and fatigue were present in 27.3% (95% CI, 16.6% to 39.4%; I2=87.6%) and 16.7% (95% CI, 10.4% to 24.2%; I2=72.5%) of patients, respectively. GI symptoms were reported by 11.6% (95% CI, 7.7% to 16.1%; I2=54.6%) of patients (Fig. 2D).
In a comparison of symptoms between the patients diagnosed in Wuhan and outside of Wuhan, fever was more frequently observed in the patients diagnosed in Wuhan (91.7%; 95% CI, 88.0% to 94.8%; I2=17.94%) than in those diagnosed outside of Wuhan (78.1%; 95% CI, 73.2% to 82.7%; I2=0%) (Fig. 3A). However, the proportion of patients with cough was similar between the Wuhan (41.3%; 95% CI, 8.2% to 79.9%; I2=97.9%) and non-Wuhan groups (59.4%; 95% CI, 47.8% to 70.4%; I2=71.2%). Similar to fever, dyspnea was significantly more frequent in the patients diagnosed in Wuhan (21.1%; 95% CI, 13.2% to 30.3%; I2=11.0%) than in those diagnosed outside of Wuhan (3.80%; 95% CI, 0.13% to 12.22%; I2=74.75%) (Fig. 3B). However, no significant difference was observed in the frequency of sputum between the Wuhan group (23.33%; 95% CI, 18.1% to 29.1%; I2=0%) and the non-Wuhan group (17.7%; 95% CI, 11.5% to 24.9%; I2=0%).
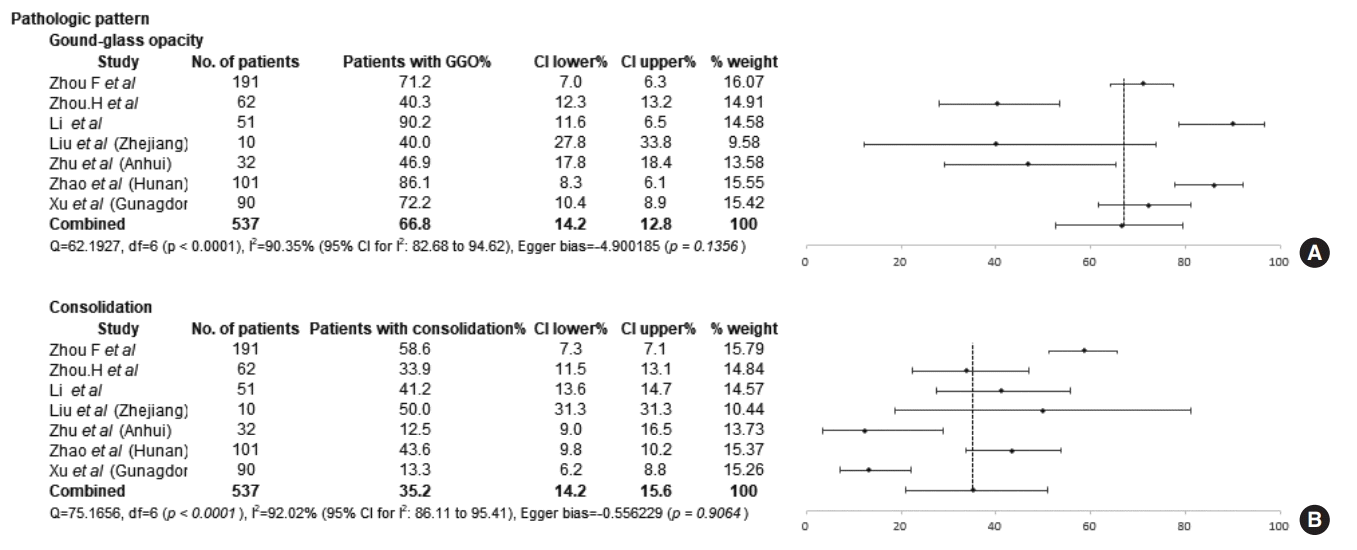
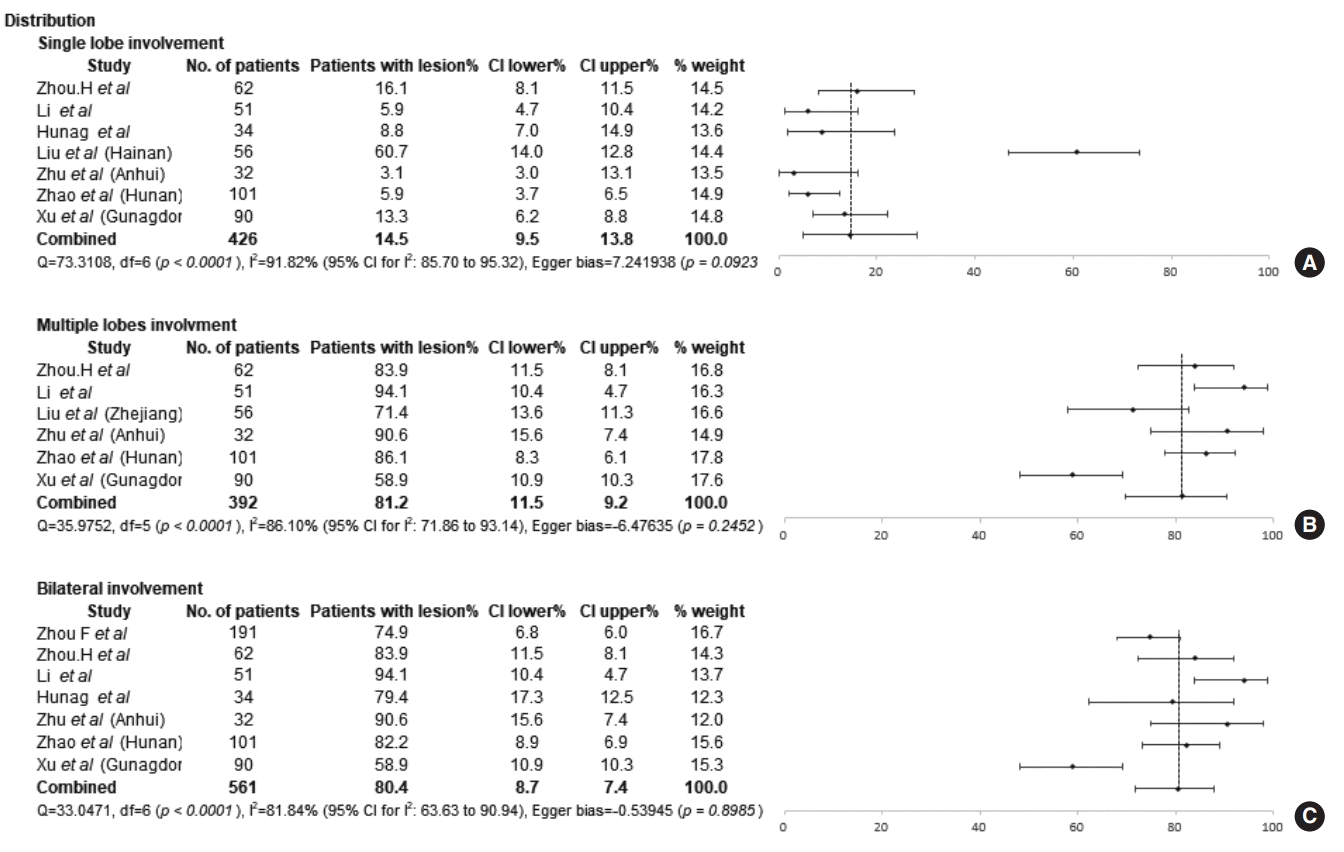
The chest CT findings of the included studies are presented in Table 3. A GGO pattern and consolidation were observed in 66.76% (95% CI, 52.56% to 79.55%; I2=90.35%) and 35.15% (95% CI, 20.96% to 50.84%; I2=92.02%) of COVID-19 patients, respectively (Fig. 4). The most common distribution of pathologic findings was involvement of multiple lobes (81.21%; 95% CI, 69.73% to 90.46%; I2=86.10%), while only 14.47% (95% CI, 4.99% to 28.27%; I2=91.82%) of patients had pathologic findings distributed in a single lobe (Fig. 5). Bilateral lung involvement was identified in 80.41% (95% CI, 71.75% to 87.81%; I2=81.84%) of patients. No significant difference was observed in the frequency of patients in whom the pathologic findings showed only single-lobe involvement between the Wuhan group (11.09%; 95% CI, 5.71% to 17.96%; I2=32.20%) and the nonWuhan group (17.77%; 95% CI, 2.22% to 43.56%; I2=95.59%). The distribution of multiple-lobe and bilateral lung involvement was similar in both groups. The results of the comparison of the proportions of chest CT findings between the groups are presented in Supplementary Figs. 1 and 2.
Within only 3 months after it was first reported in China, the COVID-19 pandemic has spread to South Korea, Iran, Italy, and the United States, and the number of patients worldwide has rapidly increased [6,7]. Since COVID-19 is estimated to be more infectious than MERS and more fatal than influenza, physicians and health-care providers should actively work to diagnose COVID-19 and to keep COVID-19 patients in isolation to minimize the spread of the disease and prevent deterioration of the current situation. Although COVID-19 is diagnosed using rRT-PCR tests, proper screening conducted by health-care providers is the most important prerequisite for controlling the spread of COVID-19, as diagnostic capacity and accessibility of medical care differ from country to country [15,42].
Patients’ symptoms are important clues for the diagnosis of a disease, and the same is true for the screening of diseases [15]. Essentially, all the relevant guidelines recommend that symptoms and epidemiological risk (history of contact with COVID-19 patients and history of visits to pandemic-affected areas) should be considered as part of COVID-19 screening [7,12,43,44]. According to the Centers for Disease Control and Prevention, screening for COVID-19 should be based on the presence of symptoms of fever, cough, and shortness of breath [12]. The most common symptom in patients diagnosed with COVID-19 was fever (84.8%; 95% CI, 78.5% to 90.1%; I2=73.6%), which was frequently accompanied by cough (52.0%; 95% CI, 34.1% to 69.7%; I2=95.0%). In addition, 21.3% and 10.4% of patients complained of sputum and dyspnea (other signs of respiratory infection). Moreover, myalgia (27.3%) and fatigue (16.7%), which are symptoms of inflammation due to SARS-CoV-2, were also less common than fever and cough. Although all of symptoms mentioned above, except sputum, exhibited significant heterogeneity, fever accompanied by the signs of a respiratory infection can be considered as a reasonable parameter for suspecting COVID-19, especially in patients with epidemiological risk, as has been recommended by many guidelines [7,12,43,44]. Interestingly, over 10% of patients in the present study complained of GI symptoms. GI symptoms are thought to be a characteristic of SARSCoV-2 infection, especially in light of the previous finding that over 30% of MERS patients complained of GI symptoms [45]. Recently, olfactory and gustatory dysfunction as determined by a questionnaire evaluation has been observed in 85% of mildto-moderate COVID-19 patients in Europe; the reported frequency of this symptom is therefore similar to that of fever and higher than that of cough in the present study [46]. Thus, physicians, especially otorhinolaryngologists, should be aware of these symptoms, in addition to fever accompanied by the signs of respiratory infection, as a possible clinical presentation of COVID-19. Olfactory and gustatory complaints were the initial symptoms in approximately 15% of COVID-19 patients, and 11.8% of patients complained of only olfactory changes without other nasal symptoms [46]. However, we could not find any information on these symptoms in the studies included in this meta-analysis. The patients included in this study were mainly diagnosed with COVID-19 in January 2020, not long after the emergence of COVID-19. Since these diagnoses were made in the early phase of COVID-19 transmission, a lack of awareness of olfactory/gustatory dysfunction, as well as differences in disease severity in patients depending on the time of COVID-19 diagnosis, may explain why information on these symptoms was missing in the included studies. Although the results of this study did not address olfactory or gustatory dysfunctions observed in COVID-19 patients, based on current knowledge, otorhinolaryngologists must consider the possibility of COVID-19 if patients with epidemiological risk of this disease complain of olfactory or gustatory dysfunction.
Fever is known to occur in a relatively early phase of COVID19, whereas dyspnea is a symptom that generally occurs in the rapid progression phase, which takes place 3 to 7 days after the onset of symptoms [44]. The present meta-analysis revealed that fever and dyspnea were less frequent in the patients diagnosed outside of Wuhan than in those diagnosed in Wuhan. In addition, asymptomatic COVID-19 patients were only reported in the non-Wuhan group. In a report from South Korea, among a total of 28 patients, three patients without symptoms (10.71%) were diagnosed with COVID-19, and only 32.14% of patients (9/28) complained of fever [47]. This trend was also observed in a study reporting the clinical features of 38 COVID-19 patients in Europe; 5.26% of patients (2/38) were asymptomatic and fever was observed in only 52.63% (20/38) [48]. Most studies of both groups (the Wuhan group and the non-Wuhan group) in this meta-analysis included patients diagnosed in a similar period. Since the initial outbreak of COVID-19 is assumed to have occurred in Wuhan, the interval between infection and COVID-19 diagnosis is thought to have been shorter in the non-Wuhan group than in the Wuhan group. Thus, the lower frequency of fever and the higher number of asymptomatic patients observed outside of Wuhan is thought to be due to the early diagnosis of COVID-19 as a consequence of physicians’ increased awareness of the disease and epidemiological assessments of high-risk individuals. Although it was not practicable to conduct a meta-regression analysis of the effect of age on clinical manifestations because of variation in how age was reported in the included studies, the patients from outside of Wuhan seemed to be younger than the patients diagnosed in Wuhan. Since the clinical manifestations and disease severity of COVID-19 vary depending on age [38], these results should be interpreted with caution, in light of the possibility that the age difference between the groups may have affected the differences in clinical manifestations found in this study.
Regarding disease screening, we still should be aware that fever and dyspnea were less frequent in patients diagnosed outside of Wuhan in China. It is apparent that only using symptoms as screening criteria for COVID-19 results in an increased chance of failure of early diagnosis of COVID-19 in actual patients. Therefore, the assessment of epidemiological risk is of prime importance in patients complaining of fever accompanied by signs of respiratory infection. If it is not possible to conduct rRT-PCR for COVID-19 in high-risk individuals, proper isolation of these patients for 2 weeks to minimize their contact with other people around them—even if they do not have symptoms or their symptoms are minor—is necessary considering the incubation period of COVID-19 [43]. In addition, it would be preferable to have a monitoring system for identifying patients’ history of visits to pandemic-affected areas and for keeping track of high-risk individuals through government interventions with the purpose of controlling this infectious disease.
Numerous studies have reported the radiologic findings of COVID-19 patients. While simple chest radiography plays a limited role in COVID-19 screening, chest CT is known to detect lung abnormalities, single or focal GGO patterns, the presence of a nodule in the central lobule, and patchy consolidation even before the onset of symptoms because of its high resolution [34,40,44]. GGO is an initial pathologic finding that can be observed in the lungs even before symptom onset, whereas the consolidation pattern is a relatively late finding in the progression of COVID-19 [44]. These pathologic findings gradually involve bilateral and multiple lobes during the course of disease progression. Although we assumed that COVID-19 was diagnosed earlier in the non-Wuhan group, no significant betweengroup differences were found in the distribution of patterns involving a single lobe, multiple lobes, and the bilateral lungs. The results regarding GGO and consolidation are similar to results reported from South Korea (GGO, 8/9; consolidation, 2/9) [49]. Since all five parameters reflecting chest CT findings exhibited considerable heterogeneity in the results extracted from the included studies, the present study does not provide support for any conclusions regarding the role of chest CT in COVID-19 screening.
This study has several limitations and the results should be interpreted with caution. First, the results of this study can only serve as the basis for weak recommendations because of the low level of evidence of the included studies, the small number of patients, and methodological limitations [24]. Second, there was considerable heterogeneity in the results of the included studies. It is hypothesized that these outcomes could reflect differences between the groups. Third, it is possible that this study did not include exhaustive information about COVID-19, as case reports and studies with insufficient information were excluded from the present analysis to increase the level of evidence.
In conclusion, fever accompanied by signs of respiratory infection is considered to be the most reliable manifestation of COVID-19. Fever and dyspnea were observed less frequently in patients diagnosed outside of Wuhan in China, which should be considered in COVID-19 screening. Regional differences in the proportion of symptoms may be attributable to the earlier diagnosis of the disease and the younger age of the patients outside of Wuhan although further analysis is needed. Finally, the results of this study do not support any specific conclusions regarding the role of chest CT in diagnosing COVID-19.
▪ Fever was found to be the most common clinical manifestation in all coronavirus disease 2019 (COVID-19) patients.
▪ Fever and dyspnea were less frequent in patients diagnosed outside of Wuhan than in those diagnosed in Wuhan, which may be attributable to the earlier diagnosis of the disease and the younger age of patients outside of Wuhan.
▪ Since there were no significant differences in pathologic patterns and their distribution on chest computed tomography (CT) between the patients diagnosed in Wuhan and outside of Wuhan, the role of chest CT in the diagnosis of COVID-19 is inconclusive based on this study.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.21053/ceo.2020.00570.
Supplementary Table 1.
Quality assessment tool for case series studies provided by the National Institutes of Health
Supplementary Table 2.
The proportion of laboratory findings in patients with COVID-19
REFERENCES
1. Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020; Apr. 92(4):401–2.

2. Shim E, Tariq A, Choi W, Lee Y, Chowell G. Transmission potential and severity of COVID-19 in South Korea. Int J Infect Dis. 2020; Mar. 93:339–44.

3. Tuite AR, Bogoch II, Sherbo R, Watts A, Fisman D, Khan K. Estimation of coronavirus disease 2019 (COVID-19) burden and potential for international dissemination of infection from Iran. Ann Intern Med. 2020; Mar. 16. [Epub]. https://doi.org/10.7326/M20-0696.

4. Saglietto A, D’Ascenzo F, Zoccai GB, De Ferrari GM. COVID-19 in Europe: the Italian lesson. Lancet. 2020; Apr. 395(10230):1110–1.

5. World Health Organization. Novel coronavirus (2019-nCoV) situation report-10 [Internet]. Geneva, CH: World Health Organization;2020 [cited 2020 Apr 22]. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200130-sitrep-10-ncov.pdf?sfvrsn=d0b2e480.
6. World Health Organization. Coronavirus disease 2019 (COVID-19) situation report-67 [Internet]. Geneva, CH: World Health Organization;2020 [cited 2020 Apr 22]. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200327-sitrep-67-covid-19.pdf?sfvrsn=b65f68eb.
7. Eurosurveillance Editorial Team. Updated rapid risk assessment from ECDC on the novel coronavirus disease 2019 (COVID-19) pandemic: increased transmission in the EU/EEA and the UK. Euro Surveill. 2020; Mar. 25(10):2003121.
8. Liu Y, Gayle AA, Wilder-Smith A, Rocklov J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020; Mar. 27(2):taaa021.

9. Callaway E, Cyranoski D, Mallapaty S, Stoye E, Tollefson J. The coronavirus pandemic in five powerful charts. Nature. 2020; Mar. 579(7800):482–3.

10. Peng L, Liu J, Xu W, Luo Q, Deng K, Lin B, et al. 2019 Novel coronavirus can be detected in urine, blood, anal swabs and oropharyngeal swabs samples [Internet]. medRxiv. 2020. [cited 2020 Apr 21]. Available from: https://doi.org/10.1101/2020.02.21.20026179.

11. Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020; Jan. 25(3):2000045.

12. Centers for Disease Control. Interim infection prevention and control recommendations for patients with suspected or confirmed coronavirus disease 2019 (COVID-19) in healthcare settings [Internet]. Atlanta, GA: Centers for Disease Control and Prevention;2020 [cited 2020 Apr 22]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/infection-control/control-recommendations.html.
13. World Health Organization. Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases [Internet]. Geneva, CH: World Health Organization;2020. [cited 2020 Apr 22]. Available from: https://www.who.int/publications-detail/laboratory-testingfor-2019-novel-coronavirus-in-suspected-human-cases-20200117.
14. Lippi G, Plebani M. The critical role of laboratory medicine during coronavirus disease 2019 (COVID-19) and other viral outbreaks. Clin Chem Lab Med. 2020; Mar. 19. [Epub]. https://doi.org/10.1515/cclm-2020-0240.

15. World Health Organization. Controlling the spread of infectious disease. In : Magnusson R, editor. Advancing the right to health: the vital role of law. Geneva, CH: World Health Organization;2016. p. 151–64.
16. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutierrez-Ocampo E, Villamizar-Pena R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020; Mar. 13. [Epub]. https://doi.org/10.1016/j.tmaid.2020.101623.

17. Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020; Mar. 12. [Epub]. https://doi.org/10.1002/jmv.25757.

18. Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma CL, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020; Feb. 368:m606.

19. Kim JY, Choe PG, Oh Y, Oh KJ, Kim J, Park SJ, et al. The first case of 2019 novel coronavirus pneumonia imported into Korea from Wuhan, China: implication for infection prevention and control measures. J Korean Med Sci. 2020; Feb. 35(5):e61.

20. Pan Y, Guan H, Zhou S, Wang Y, Li Q, Zhu T, et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. 2020; Feb. 13. [Epub]. https://doi.org/10.1007/s00330-020-06731-x.

21. Titler MG. The evidence for evidence-based practice implementation. In : Hughes RG, editor. Patient safety and quality: an evidence-based handbook for nurses. Rockville, MD: Agency for Healthcare Research and Quality, US;2008. p. 113–61.
22. Murad MH, Asi N, Alsawas M, Alahdab F. New evidence pyramid. Evid Based Med. 2016; Aug. 21(4):125–7.

23. Murad MH. Clinical practice guidelines: a primer on development and dissemination. Mayo Clin Proc. 2017; Mar. 92(3):423–33.
24. Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018; Apr. 23(2):60–3.

25. Abu-Zidan FM, Abbas AK, Hefny AF. Clinical “case series”: a concept analysis. Afr Health Sci. 2012; Dec. 12(4):557–62.

26. Bauchner H, Golub RM, Zylke J. Editorial concern-possible reporting of the same patients with COVID-19 in different reports. JAMA. 2020; Mar. 323(13):1256.

27. National Heart, Lung, and Blood Institute; National Institutes of Health. Quality assessment tool for case series studies [Internet]. Bethesda, MA: National Heart, Lung, and Blood Institute;2018. [cited 2020 Apr 22]. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
28. El Dib R, Nascimento Junior P, Kapoor A. An alternative approach to deal with the absence of clinical trials: a proportional meta-analysis of case series studies. Acta Cir Bras. 2013; Dec. 28(12):870–6.

29. Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0: identifying and measuring heterogeneity [Internet]. West Sussex, UK: John Wiley & Sons;2011. [cited 2020 Apr 22]. Available from: https://handbook-5-1.cochrane.org/chapter_9/9_5_2_identifying_and_measuring_heterogeneity.htm.
30. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; Sep. 327(7414):557–60.

31. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; Sep. 315(7109):629–34.

32. Neyeloff JL, Fuchs SC, Moreira LB. Meta-analyses and Forest plots using a microsoft excel spreadsheet: step-by-step guide focusing on descriptive data analysis. BMC Res Notes. 2012; Jan. 5:52.

33. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020; Mar. 395(10229):1054–62.

34. Zhou S, Wang Y, Zhu T, Xia L. CT features of coronavirus disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. AJR Am J Roentgenol. 2020; Mar. 5. [Epub]. https://doi.org/10.2214/AJR.20.22975.

35. Li Y, Xia L. Coronavirus disease 2019 (COVID-19): role of chest CT in diagnosis and management. AJR Am J Roentgenol. 2020; Mar. 4. [Epub]. https://doi.org/10.2214/AJR.20.22954.

36. Huang Y, Tu M, Wang S, Chen S, Zhou W, Chen D, et al. Clinical characteristics of laboratory confirmed positive cases of SARS-CoV-2 infection in Wuhan, China: a retrospective single center analysis. Travel Med Infect Dis. 2020; Feb. 27. [Epub]. https://doi.org/10.1016/j.tmaid.2020.101606.

37. Liu F, Xu A, Zhang Y, Xuan W, Yan T, Pan K, et al. Patients of COVID19 may benefit from sustained lopinavir-combined regimen and the increase of eosinophil may predict the outcome of COVID-19 progression. Int J Infect Dis. 2020; Mar. 6. [Epub]. https://doi.org/10.1016/j.ijid.2020.03.013.

38. Liu K, Chen Y, Lin R, Han K. Clinical features of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J Infect. 2020; Mar. 11. [Epub]. https://doi.org/10.1016/j.jinf.2020.03.005.

39. Zhu W, Xie K, Lu H, Xu L, Zhou S, Fang S. Initial clinical features of suspected coronavirus disease 2019 in two emergency departments outside of Hubei, China. J Med Virol. 2020; Mar. 13. [Epub]. https://doi.org/10.1002/jmv.25763.

40. Zhao W, Zhong Z, Xie X, Yu Q, Liu J. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. AJR Am J Roentgenol. 2020; Mar. [Epub]. https://doi.org/10.2214/AJR.20.22976.

41. Xu X, Yu C, Qu J, Zhang L, Jiang S, Huang D, et al. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. 2020; May. 47(5):1275–80.

42. Quinn SC, Kumar S. Health inequalities and infectious disease epidemics: a challenge for global health security. Biosecur Bioterror. 2014; Sep-Oct. 12(5):263–73.

43. Central Accident Investigation Headquarters. Korean Government’s Response System [Internet]. Sejong, KR: Central Accident Investigation Headquarters;2020 [cited 2020 Apr 22]. Available from: http://ncov.mohw.go.kr/en/baroView.do?brdId=11&brdGubun=111&dataGubun=&ncvContSeq=&contSeq=&board_id.
44. Jin YH, Cai L, Cheng ZS, Cheng H, Deng T, Fan YP, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res. 2020; Feb. 7(1):4.

45. Arabi YM, Balkhy HH, Hayden FG, Bouchama A, Luke T, Baillie JK, et al. Middle East respiratory syndrome. N Engl J Med. 2017; Feb. 376(6):584–94.

46. Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020; Apr. 6. [Epub]. https://doi.org/10.1007/s00405-020-05965-1.

47. COVID-19 National Emergency Response Center; Epidemiology and Case Management Team; Korea Centers for Disease Control and Prevention. Early epidemiological and clinical characteristics of 28 cases of coronavirus disease in South Korea. Osong Public Health Res Perspect. 2020; Feb. 11(1):8–14.
Table 1.
Brief information about the included studies
| First author | Hospital | Province | Level of evidence | Date of patients’ inclusion | No. of patients | Outcome |
Quality assessment |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Total | ||||||||
| Wuhan | 338 | ||||||||||||||||
| Zhou [33] | Jinyintan Hospital | Hubei | IV | 01/16/2020 | 191 | S, CT, L | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good | |
| Wuhan Pulmonary Hospital | |||||||||||||||||
| Zhou [34] | Huazhong University Hospital | Hubei | IV | 12/29/2019 | 62 | S, CT, L | Yes | Yes | Yes | Yes | Yes | Yes | NA | NA | Yes | Good | |
| Li [35] | Tongji Hospital | Hubei | IV | 01/16/2020 | 51 | S, CT | Yes | Yes | Yes | No | Yes | Yes | NA | CD | Yes | Good | |
| Huang [36] | Zhongnan Hospital | Hubei | IV | 01/23/2020 | 34 | S, CT, L | Yes | Yes | NA | NA | Yes | Yes | No | NA | Yes | Fair | |
| Outside of Wuhan | 289 | ||||||||||||||||
| Liu [37] | Xixi Hospital | Zhejiang | IV | 01/22/2020 | 10 | S, CT, L | Yes | Yes | Yes | NA | Yes | Yes | Yes | NA | Yes | Good | |
| Liu [38] | Hainan Provincial People’s Hospital | Hainan | IV | 01/15/2020 | 56 | S, CT, L | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good | |
| Zhu [39] | First Affiliated Hospital of USTC | Anhui | IV | 01/24/2020 | 32 | S, CT, L | Yes | Yes | Yes | Yes | Yes | Yes | NA | NA | Yes | Good | |
| Zhao [40] | Changsha Hospital | Hunan | IV | - | 101 | S, CT | Yes | Yes | Yes | NA | No | Yes | NA | Yes | Yes | Fair | |
| Yueyang Hospital | |||||||||||||||||
| Changde Hospital | |||||||||||||||||
| Xiangtan Hospital | |||||||||||||||||
| Xu [41] | Guangzhou Eighth People’s Hospital | Guangdong | IV | 01/23/2020 | 90 | S, CT | Yes | Yes | Yes | NA | Yes | Yes | NA | NA | Yes | Fair | |
| Total | 627 | ||||||||||||||||
Table 2.
The proportions of clinical features of the included subjects
| First author | Age (range, yr) | Sex (M:F) | No. of patients |
Symptom, no. (%) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| None | Fever | Cough | Sputum | Dyspnea | Myalgia | Sore throat | Headache | GI | Fatigue | |||||
| Wuhan | ||||||||||||||
| Zhou [33] | Median, 56.0 (46–57) | 119:72 | 191 | 180 (94.2) | 151 (79.0) | 44 (23.0) | 29 (15.2) | 16 (8.3) | 44 (23.0) | |||||
| Zhou [34] | Mean, 52.8 (30–77) | 39:23 | 62 | 54 (87.1) | 28 (45.2) | 15 (24.2) | 20 (32.2) | 9 (14.5) | 14 (22.6) | |||||
| Li [35] | Mean, 58.0 (26–83) | 28:23 | 51 | 1 (2.0) | 46 (90.2) | 1 (2.0) | 3 (5.9) | |||||||
| Huang [36] | Mean, 56.0 (26–88) | 14:20 | 34 | 32 (94.1) | 17 (30) | 8 (23.5) | 5 (14.7) | 22 (64.7) | 2 (5.9) | 5 (14.7) | ||||
| Total | 200:138 | 338 | 1/51 (2.0) | 312/338 (92) | 197/338 (58.3) | 52/225 (23.1) | 20/96 (20.8) | 71/287 (24.7) | 2/34 (5.9) | 30/287 (10.5) | 61/304 (20.1) | |||
| Outside of Wuhan | ||||||||||||||
| Liu [37] | Median, 42 (34–50) | 4:6 | 10 | 7 (70.0) | 8 (80.0) | 4 (40.0) | 3 (30.0) | 3 (30.0) | ||||||
| Liu [38] | NA | 31:25 | 56 | 44 (78.6) | 21 (37.5) | 4 (7.1) | 10 (17.9) | 5 (8.9) | ||||||
| Zhu [39] | Median, 46 (35–52) | 15:17 | 32 | 27 (84.4) | 21 (66.6) | 5 (15.6) | 5 (15.6) | 1 (3.1) | 1 (3.1) | |||||
| Zhao [40] | Mean, 44.44 (17–75) | 56:45 | 101 | 2 (2.1) | 79 (78.2) | 63 (62.4) | 1 (1.0) | 17 (16.8) | 12 (11.9) | 5 (5.0) | ||||
| Xu [41] | Median, 50 (18–86) | 39:51 | 90 | 6 (6.7) | 70 (77.8) | 57 (63.3) | 11 (12.2) | 25 (27.8) | 23 (25.6) | 4 (4.4) | 12 (13.3) | 19 (21.1) | ||
| Total | 145:144 | 289 | 8/191 (4.2) | 227/289 (78.5) | 170/289 (58.8) | 16/122 (13.1) | 5/157 (6.3) | 47/223 (21.1) | 39/201 (19.4) | 8/132 (6.1) | 31/289 (10.7) | 24/146 (16.4) | ||
| Total | 345:282 | 627 | 9/242 (4.1) | 539/627 (86.0) | 367/627 (58.5) | 68/347 (19.6) | 25/253 (9.9) | 118/510 (22.9) | 39/201 (19.4) | 10/166 (6.0) | 61/576 (10.6) | 85/450 (18.9) | ||
Table 3.
The proportions of pathologic patterns and their distributions on chest computed tomography of the included subjects
| First author | No. of patients |
Pathologic pattern |
Distribution |
|||
|---|---|---|---|---|---|---|
| GGO | Consolidation | Single lobe | Multiple lobe | Both lungs | ||
| Wuhan | ||||||
| Zhou [33] | 191 | 136 (71.2) | 112 (58.6) | 143 (74.8) | ||
| Zhou [34] | 62 | 25 (40.3) | 21 (33.8) | 10 (16.1) | 52 (83.8) | 52 (83.9) |
| Li [35] | 51 | 46 (90.2) | 21 (41.1) | 3 (5.9) | 48 (94.1) | 48 (94.1) |
| Huang [36] | 34 | 3 (8.8) | 27 (79.4) | |||
| Total | 338 | 207/304 (68.1) | 154/304 (50.6) | 16/147 (10.8) | 100/113 (88.5) | 270/338 (79.9) |
| Outside of Wuhan | ||||||
| Liu [37] | 10 | 4 (40.0) | 5 (50.0) | |||
| Liu [38] | 56 | 34 (60.7) | 40 (71.4) | |||
| Zhu [39] | 32 | 15 (46.9) | 4 (12.5) | 1 (3.1) | 29 (90.6) | 29 (90.6) |
| Zhao [40] | 101 | 87 (86.1) | 44 (43.6) | 6 (5.9) | 87 (86.1) | 83 (82.1) |
| Xu [41] | 90 | 65 (72.2) | 12 (13.3) | 12 (13.3) | 53 (58.9) | 53 (58.8) |
| Total | 289 | 171/233 (73.3) | 65/233 (27.9) | 53/279 (19.0) | 209/279 (74.9) | 165/223 (74.0) |
| Total | 627 | 378/537 (66.8) | 219/537 (40.8) | 383/561 (68) | 309/392 (78.8) | 435/561 (77.5) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download