1. Chae MP, Rozen WM, Whitaker IS, Chubb D, Grinsell D, Ashton MW, et al. Current evidence for postoperative monitoring of microvascular free flaps: a systematic review. Ann Plast Surg. 2015; May. 74(5):621–32.
2. Buncke HJ. Microsurgery: transplantation-replantation, an atlas text. Philadelphia (PA): Lea & Febiger;1991.
3. Disa JJ, Cordeiro PG, Hidalgo DA. Efficacy of conventional monitoring techniques in free tissue transfer: an 11-year experience in 750 consecutive cases. Plast Reconstr Surg. 1999; Jul. 104(1):97–101.

4. Jones BM. Monitors for the cutaneous microcirculation. Plast Reconstr Surg. 1984; May. 73(5):843–50.

5. Pellini R, Pichi B, Marchesi P, Cristalli G, Deganello A, Spriano G. External monitor for buried free flaps in head and neck reconstructions. Acta Otorhinolaryngol Ital. 2006; Feb. 26(1):1–6.
6. Ferguson RE Jr, Yu P. Techniques of monitoring buried fasciocutaneous free flaps. Plast Reconstr Surg. 2009; Feb. 123(2):525–32.

7. Yu P, Robb GL. Pharyngoesophageal reconstruction with the anterolateral thigh flap: a clinical and functional outcomes study. Plast Reconstr Surg. 2005; 116(7):1845–55.

8. Yu P, Hanasono MM, Skoracki RJ, Baumann DP, Lewin JS, Weber RS, et al. Pharyngoesophageal reconstruction with the anterolateral thigh flap after total laryngopharyngectomy. Cancer. 2010; Apr. 116(7):1718–24.

9. Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011; Mar-Apr. 10(2):150–61.

10. Lindau RH, Detwiller K, Wax MK. Buried free flaps in head and neck surgery: outcome analysis. Head Neck. 2013; Oct. 35(10):1468–70.

11. Kim SY, Kang CH, Park IK, Kim YT. Esophageal stent insertion for postesophagectomy anastomosis site leakage. Clin Exp Otorhinolaryngol. 2016; Dec. 9(4):382–4.

12. Haksever M, Akduman D, Aslan S, Solmaz F, Ozmen S. Modified continuous mucosal Connell suture for the pharyngeal closure after total laryngectomy: zipper suture. Clin Exp Otorhinolaryngol. 2015; Sep. 8(3):281–8.

13. Pirracchio R, Resche-Rigon M, Chevret S. Evaluation of the propensity score methods for estimating marginal odds ratios in case of small sample size. BMC Med Res Methodol. 2012; May. 12:70.

14. Chang EI, Zhang H, Liu J, Yu P, Skoracki RJ, Hanasono MM. Analysis of risk factors for flap loss and salvage in free flap head and neck reconstruction. Head Neck. 2016; Apr. 38 Suppl 1:E771–5.

15. Kim H, Jeong WJ, Ahn SH. Results of free flap reconstruction after ablative surgery in the head and neck. Clin Exp Otorhinolaryngol. 2015; Jun. 8(2):167–73.





 PDF
PDF Citation
Citation Print
Print



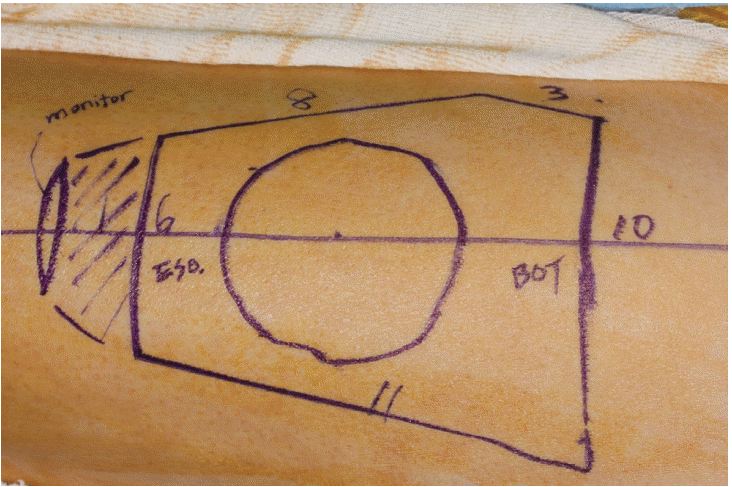
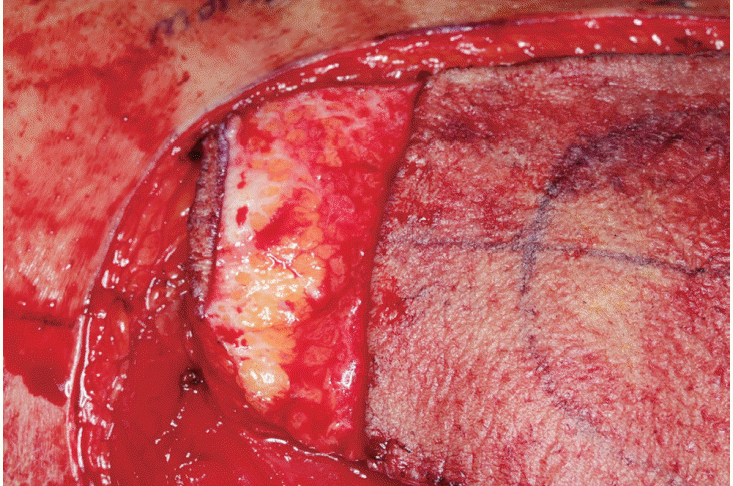
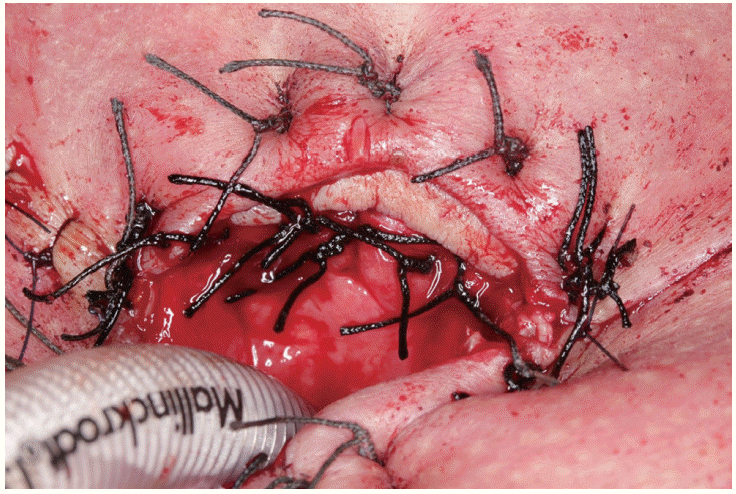
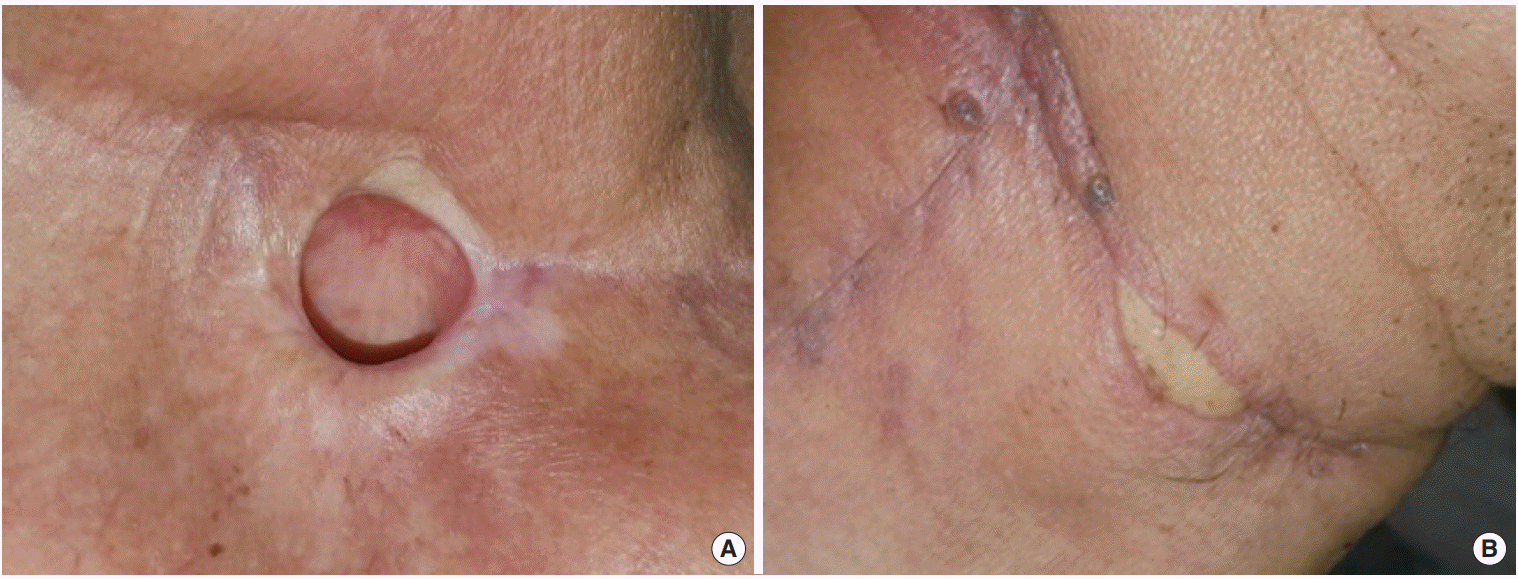
 XML Download
XML Download