Abstract
Objectives
Postinfectious olfactory dysfunction (PIOD) is the most common etiology of olfactory dysfunction, and olfactory training (OT) is an accepted treatment modality for PIOD. Some studies have investigated OT in Korean patients, but they involved odorants unfamiliar to Koreans or had no control group. The aim of this study was to verify the efficacy of OT in PIOD patients, using odorants familiar to Koreans and including a control group.
Methods
We enrolled a total of 104 Korean patients with PIOD over the 3-year study period. All participants were assessed using endoscopy and an olfactory function test at the baseline assessment and 3 months after OT. The olfactory function test was performed using the Korean version of Sniffin’ stick (KVSS) II. Nasal and psychological function was evaluated using a visual analog scale and the Mini-Mental State Examination. OT was performed over a period of 3 months, using five odorants (rose, lemon, cinnamon, orange, and peach).
Results
OT improved olfactory function in approximately 40% of subjects over a period of 12 weeks compared to non-OT subjects. A comparison of changes between the initial and follow-up assessments demonstrated that the OT group had significantly better olfactory results for the total KVSS II, threshold, and identification scores than the non-OT group. The degree of olfactory improvement after OT was affected by the initial score.
Olfactory dysfunction is a problem with various etiologies, including upper respiratory tract infection (URI), sinonasal disease, traumatic brain injury, and congenital anosmia [1]. Nonetheless, despite the diversity of potential causes, postinfectious olfactory loss is one of the most common etiologies of olfactory disorders [2,3]. Sudden olfactory loss may occur after an infection of the upper respiratory tract, such as the common cold or influenza [4]. Fortunately, spontaneous recovery is relatively common in postinfectious olfactory dysfunction (PIOD) patients, with reported spontaneous recovery rates varying from 6% to 35%. Thus, about one-third of PIOD patients exhibit improved olfactory function after 1 year [4]. So far, no validated medication has emerged for post-URI olfactory dysfunction, although several trials have been attempted involving systemic and topical steroids, vitamin B, caroverine, minocycline, acupuncture, and alpha-lipoic acid [5].
Individuals affected by olfactory loss can be treated through olfactory training (OT) involving repeated daily exposure to a range of odorants. In 2009, Hummel et al. [6] prospectively studied the utility of OT in a group of patients with olfactory loss due to postinfectious, posttraumatic, or idiopathic etiologies. They performed OT using four odors (phenyl ethyl alcohol, rose; eucalyptol, eucalyptus; citronella, lemon; and eugenol, cloves), which are representative of four odor categories on the odor prism proposed by Henning [6]. However, Koreans are not familiar with the odors of eucalyptus and cloves; as such, those odors were designed for Western patients. In this study, OT was performed in people with PIOD using five odorants familiar to Koreans. The use of familiar odorants may improve patients’ adherence with OT.
A limitation of previous studies of OT in Koreans is that they did not include control groups. Therefore, the aim of this study was to verify the efficacy of OT in Korean patients with PIOD, using five odorants familiar to Koreans and including a comparison with a control group.
All participants were enrolled from another institution in the Department of Otorhinolaryngology. This study was performed according to the principles of the Declaration of Helsinki. It was reviewed by Institutional Review Board of Konkuk University Medical Center (IRB No. KUH 11100063), and written informed consent was obtained from all patients. The design of the study was approved by the ethics committee of the medical faculty at Konkuk University Medical Center. A total of 104 patients with olfactory dysfunction were enrolled (79 women, 25 men; mean age, 56.5±13.0 years; range, 12–88 years).
In total, 104 patients out of 108 were included in this study over the 3-year study period. Those with olfactory dysfunction and a recent history of URI were included in the study. Exclusion criteria included pregnancy, chronic rhinosinusitis, the presence of a malignant tumor and/or a history of treatment for a malignant tumor (e.g., radiation and chemotherapy), a history of nasal surgery, hypothyroidism, and liver cirrhosis. Patients whose patterns of threshold and identification score change were opposite were also excluded. We only included patients whose follow-up period was 12±2 weeks. The Mini-Mental State Examination (MMSE) [7] was performed to screen for major cognitive impairment in patients older than 55 years. Patients with preclinical stage of dementia were excluded.
The medical history of all participants was recorded with a standardized form at the first visit. A systemic examination including endoscopy of the olfactory cleft was performed. Olfactory dysfunction was classified as postinfectious olfactory loss depending on the clinical findings and past medical history. The olfactory function test was performed using the Korean version of Sniffin’ stick (KVSS) II. Nasal and psychological function was evaluated using a visual analog scale and the MMSE. All participants were reassessed using endoscopy and the KVSS II at 3 months after the baseline assessment.
OT was performed over a period of 3 months. The patients were exposed twice a day to five odorants: rose [6], lemon [6], cinnamon, orange [8], and peach [9]. These odorants were chosen as representative of familiar odorants for Koreans [10,11]. The odors were not only based on single molecule but mixtures of odorants. The patients received five plastic bottles, labelled with the name of odor (total volume, 10 mL). They were asked to sniff the odorants for 10 seconds each in the morning and evening, with a time interval of 10 seconds between each odorant. They kept a diary in which they recorded their overall olfactory ability every day. Patients in the non-training group were told to wait and see how the spontaneous recovery of olfactory function would occur.
We provided counseling on behavior and lifestyle modifications to all patients with olfactory dysfunction. For example, installing gas and smoke detectors was recommended to protect against fire. Second, labeling of food expiration dates and spices and colors in food to enhance enjoyment was advised. Third, attention to personal hygiene and other psychological aids was included.
For statistical analyses, SPSS ver. 14.0 (SPSS Inc., Chicago, IL, USA) was used. Comparisons between the two groups were performed using independent t-test and chi-square tests. Analysis of variance (ANOVA; measures design: RM-ANOVA) was used for comparisons of olfactory function (within-subject-factor: Sniffin’ Sticks subtest) between groups (between-subject-factor: group [training, no training]) obtained before and after a period during which some of the subjects trained while others did not (session: before, after). Age at diagnosis, sex and duration of PIOD were used as covariates.
Correlation analyses were performed according to Pearson to investigate the relation between changes in threshold, discrimination, and identification (TDI) scores and subjects’ age, sex, duration of PIOD or initial KVSS II score. The alpha level was set at 0.05.
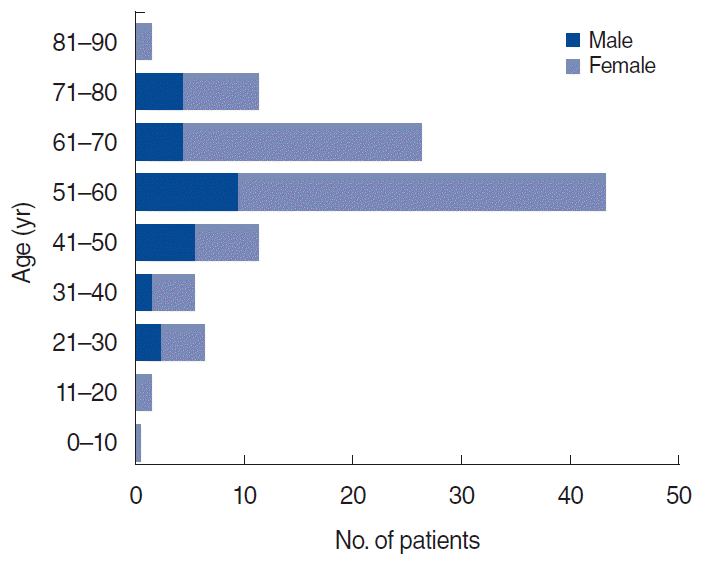
This study included 104 participants (mean age, 56.5±13.0 years; range, 12–88 years). The OT group consisted of 40 patients (mean age, 55.7 years; 30 females and 10 males; duration of disorder, 6.1 months) and the non-OT group comprised 64 patients (mean age, 57.0 years; 49 females and 15 males; duration of disorder, 7.4 months). There were more female than male subjects overall, but the age and sex distribution in the two groups was similar. In terms of the sex-specific age distribution, the number of women in their 50s was the greatest (Fig. 1). We investigated lifestyle factors that could affect participants’ sense of smell, such as smoking and drinking. At the baseline assessment, there were no significant differences between the OT and non-OT groups in terms of age (P=0.62), sex distribution (P=0.85), smoking (P=1.00), drinking (P=0.49), and duration of the disorder (months; P=0.50).
Among all participants, the mean baseline TDI score was 16.4±6.3. The mean score improved slightly to 19.8±6.3 at follow-up. The mean TDI score in the OT group was 17.5±6.1 at baseline and rose to 22.1±6.8 at follow-up. In contrast, the mean TDI score in the non-OT group was 15.6±6.5 at baseline, and increased to 18.3±5.6 at follow-up. At the time of diagnosis, the groups did not differ significantly in the initial total KVSS II score (P=0.14), threshold score (P=0.68), discrimination score (P=0.18), or identification score (P=0.11). In the final olfactory function test, the two groups showed significant differences in the total score (P=0.003), threshold score (P=0.003), and identification score (P=0.008), but not in the discrimination score (P=0.46) (Table 1).
When comparing the effects of OT between the two groups using RM-ANOVA, age, sex and duration were covariates. A comparison of TDI scores between the initial assessment and follow-up assessment demonstrated that the OT group had significantly better olfactory results on the total KVSS II (F[1, 165.35]=5.790, P=0.018), threshold score (F[1, 41.63]=5.944, P=0.017), and identification score (F[1, 34.075]=1.193, P=0.032) than the non-OT group. This indicates a positive effect of OT. There was no significant difference in the discrimination score (F[1, 34.075]=1.193, P=0.032) (Fig. 2).
In all patients (n=108), a significant correlation was found between the initial KVSS II score and the KVSS II score change (P=0.000, unstandardized coefficients=0.502). However, age, sex, and duration of the disorder had no significant effect on changes in the KVSS II score (Table 2).
PIOD is the most common etiology of olfactory dysfunction. According to several publications, OT is an accepted treatment modality for PIOD. A recent meta-analysis suggested that OT is a promising therapeutic treatment for patients with olfactory dysfunction, and most other studies have reported that OT had positive outcomes with regard to olfaction, without significant adverse effects [12].
In patients with olfactory loss with postinfectious, posttraumatic, or idiopathic etiologies, Hummel et al. [6] investigated the utility of OT for 12 weeks. In their series, 28% of the training group showed improved smell sensations in the olfactory test compared to only 6% of the control group. The most commonly studied OT protocol involves four odors, one from each of the following categories: fruity, flowery, resinous, and spicy [13]. Some studies have investigated OT in Korea, but they used odorants unfamiliar to Koreans or proceeded without a control group. A recent study explored the efficacy of OT in Chinese PIOD patients, but this study also had no control group [14]. In this study, we evaluated the effect of OT in PIOD patients using odorants familiar to Koreans and found that more of the trained patients exhibited an improvement in olfactory function after OT when compared to a control group. The current investigation showed the following major results: (1) OT increased olfactory function in approximately 40% of the subjects over a period of 12 weeks compared to subjects without OT; and (2) the degree of olfactory improvement after OT was affected by the initial score.
A recent meta-analysis showed that OT has significant positive effects on odor threshold, discrimination, identification, and the composite TDI score. The authors found a large effect on the composite TDI score as well as identification and discrimination scores, but a small to moderate effect on the threshold score [13]. Most previous studies reported that OT in PIOD resulted in improvements in the composite TDI, identification, and discrimination scores. In a prior study, although an improvement in the threshold for odor detection was observed after OT, it was not statistically significant [15]. In our study, OT lead to olfactory improvement in the composite TDI score, threshold score, and identification score in comparison to a control group. Identification and discrimination score changes after OT are related to modulation at the level of the central olfactory system or changes in the cognitive processing of olfactory information. Odor discrimination and identification are more closely correlated with tests of cognition, and identification is known to be impaired in cases of central olfactory dysfunction. As our OT kit contained odorants familiar to Koreans, the use of familiar smells may have resulted in a better identification score. In our study, statistically significant improvement of the threshold score after OT was found. The threshold score is related more closely to peripheral changes in the olfactory system (e.g., olfactory epithelium), as the odor threshold appears to be relatively unimpaired in central causes of olfactory dysfunction (e.g., focal cerebral excision) and is poorly correlated with tests of cognition. The pathophysiology of PIOD can be explained by damage at the level of the olfactory neuroepithelium. Direct transmission of pathogens to the brain via the olfactory nerve is thought to be possible [13]. Spontaneous rates of recovery as high as 35% over 1 year have been reported in patients with PIOD [12]. OT had an influence on postinfectious patients’ olfactory sensitivity (T score) by increasing the number of odor receptors or increasing the volume of the olfactory bulb [16]. However, it is also possible for patients to recover their olfactory function spontaneously over time, not through OT.
We investigated factors affecting olfactory function after OT, such as initial score, disease duration, sex, and age. Initial score was a factor affecting olfactory function after OT. Anosmia at the initial assessment was associated with improved olfactory function. This result is similar to that reported by Fleiner et al. [17]. In their study, the TDI score increased after OT in patients with baseline anosmia. A study by Damm et al. [5] found a negative correlation between recovery rate and duration of the disease. However, we could not establish a correlation between duration of the disease and the improvement of olfactory function. Calculating the duration of the disease is difficult because people may catch colds several times. Age and sex were not found to be associated with changes in olfactory function in this study or in other studies.
There are some limitations to our study. First, it had a small sample size. However, a single doctor followed up all patients from diagnosis through treatment over the study period, and also analyzed all their data. Second, this study was not a randomized controlled study, and the patients decided whether to participate in OT. This study also did not use a sham test. In principle, the control group should have engaged in OT with odor-free bottles to obtain more valid results. However, in practice, it was impossible to perform sham OT using odorless bottles. Third, our diagnoses of olfactory dysfunction as PIOD were subject to limitations in the accuracy of the etiological classification. As we depend on taking a history from the patient, it remains difficult to determine whether olfactory dysfunction has only a postinfectious cause. Additional studies should assess patients with olfactory dysfunction of other origins (e.g., sinus origin or post-trauma). Finally, this study did not make a comparison between subjects who used the odorants from the study by Hummel et al. [6] and subjects who used odorants familiar to Koreans.
In conclusion, the effectiveness of OT in patients with PIOD has been demonstrated in this study. This result is meaningful in that Korean patients were trained using odors familiar to them and that a comparison was made with a control group.
▪ Olfactory training (OT) in patients with postinfectious olfactory dysfunction resulted in significant improvements in olfactory function, as shown by higher total Korean version of Sniffin’ stick II, threshold, and identification scores than were observed in the non-OT group.
▪ The degree of olfactory improvement after OT was affected by the initial score.
▪ Koreans were trained using odors familiar to them and a control group was included.
ACKNOWLEDGMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2016R1D1A1B01-012705, NRF-2016R1A5A2012284).
REFERENCES
1. Nordin S, Bramerson A. Complaints of olfactory disorders: epidemiology, assessment and clinical implications. Curr Opin Allergy Clin Immunol. 2008; Feb. 8(1):10–5.

2. Deems DA, Doty RL, Settle RG, Moore-Gillon V, Shaman P, Mester AF, et al. Smell and taste disorders, a study of 750 patients from the University of Pennsylvania Smell and Taste Center. Arch Otolaryngol Head Neck Surg. 1991; May. 117(5):519–28.

3. Damm M, Temmel A, Welge-Lussen A, Eckel HE, Kreft MP, Klussmann JP, et al. Olfactory dysfunctions: epidemiology and therapy in Germany, Austria and Switzerland. HNO. 2004; Feb. 52(2):112–20.
5. Damm M, Pikart LK, Reimann H, Burkert S, Goktas O, Haxel B, et al. Olfactory training is helpful in postinfectious olfactory loss: a randomized, controlled, multicenter study. Laryngoscope. 2014; Apr. 124(4):826–31.

6. Hummel T, Rissom K, Reden J, Hahner A, Weidenbecher M, Huttenbrink KB. Effects of olfactory training in patients with olfactory loss. Laryngoscope. 2009; 119(3):496–9.

7. Cockrell JR, Folstein MF. Mini-Mental State Examination (MMSE). Psychopharmacol Bull. 1988; 24(4):689–92.
8. Altundag A, Cayonu M, Kayabasoglu G, Salihoglu M, Tekeli H, Saglam O, et al. Modified olfactory training in patients with postinfectious olfactory loss. Laryngoscope. 2015; Aug. 125(8):1763–6.

9. Takagi SF. A standardized olfactometer in Japan: a review over ten years. Ann N Y Acad Sci. 1987; 510:113–8.
10. Kim JM, Jeong MS, Shin DH, Seol JH, Hong SC, Cho JH, et al. Olfactory identification test using familiar distracters for Koreans. Clin Exp Otorhinolaryngol. 2014; Mar. 7(1):19–23.

11. Saito S, Ayabe-Kanamura S, Takashima Y. A smell test based on odor classification of Japanese people. Chem Senses. 1995; Jun. 20(3):379.
12. Pekala K, Chandra RK, Turner JH. Efficacy of olfactory training in patients with olfactory loss: a systematic review and meta-analysis. Int Forum Allergy Rhinol. 2016; Mar. 6(3):299–307.

13. Whitcroft KL, Hummel T. Clinical diagnosis and current management strategies for olfactory dysfunction: a review. JAMA Otolaryngol Head Neck Surg. 2019; July. 145(9):846–53.
14. Qiao XF, Wang GP, Li X, Bai YH, Zheng W. Analysis of the clinical effect of olfactory training on olfactory dysfunction after upper respiratory tract infection. Acta Otolaryngol. 2019; Jul. 139(7):643–6.

15. Sorokowska A, Drechsler E, Karwowski M, Hummel T. Effects of olfactory training: a meta-analysis. Rhinology. 2017; Mar. 55(1):17–26.

Fig. 2.
Comparison of the total threshold, discrimination, and identification (TDI) score (A), threshold score (B), discrimination score (C), identification score (D), and difference between the initial and final assessments in the olfactory training (OT) and non-OT groups. KVSS, Korean version of Sniffin’ stick. *P<0.05.
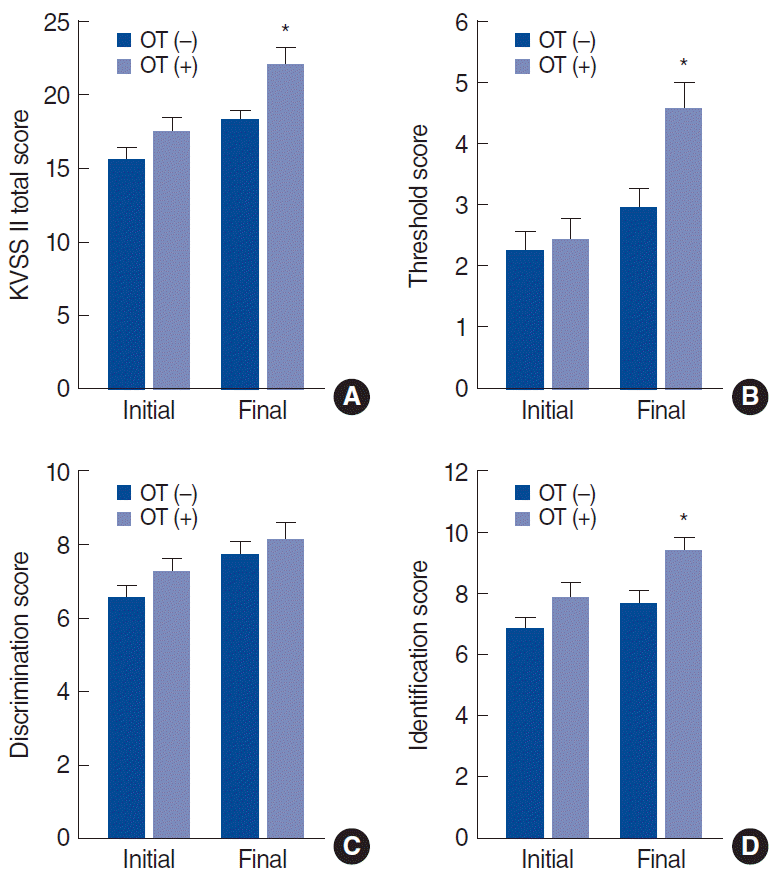
Table 1.
Demographics of patients with an olfactory disturbance
| Variable | All (n=104) | OT(+) group (n=40) | OT(–) group (n=64) | P-valuea) |
|---|---|---|---|---|
| Age (yr) | 56.5±13.0 | 55.7±12.7 | 57.0±13.3 | 0.62 |
| Female:male | 79:25 | 30:10 | 49:15 | 0.85 |
| Smoking (yes:no) | 10:94 | 4:36 | 6:58 | 1.00b) |
| Drinking (yes:no) | 30:74 | 10:30 | 20:44 | 0.49 |
| Duration (mo) | 6.9±9.5 | 6.1±10.7 | 7.4±8.7 | 0.50 |
| KVSS II test score (initial) | 16.4±6.3 | 17.5±6.1 | 15.6±6.5 | 0.14 |
| Threshold score | 2.3±2.3 | 2.4±2.1 | 2.2±2.5 | 0.68 |
| Discrimination score | 6.8±2.6 | 7.2±2.3 | 6.5±2.7 | 0.18 |
| Identification score | 7.2±3.1 | 7.8±3.3 | 6.8±2.9 | 0.11 |
| KVSS II test score (FU) | 19.8±6.3 | 22.1±6.8 | 18.3±5.6 | 0.003 |
| Threshold score | 3.6±2.7 | 4.5±2.8 | 2.9±2.4 | 0.003 |
| Discrimination score | 7.8±2.6 | 8.1±2.8 | 7.7±2.5 | 0.46 |
| Identification score | 8.3±3.2 | 9.4±2.9 | 7.6±3.2 | 0.008 |
Table 2.
Factors affecting the change of olfactory function




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download