Abstract
Objectives
To investigate the influence of pretreatment primary tumor or nodal photopenia (PP) on 18F-fluorodeoxyglucose positron emission tomography-computed tomography (FDG PET-CT), an indicator of tumor ischemia, on survival results of nasopharyngeal cancers (NPCs) treated with concurrent chemoradiotherapy (C-CRT).
Methods
The pre-C-CRT FDG PET-CT scans of 104 patients with NPC (cT1-4 N0-3 M0) were retrospectively examined to determine the presence of PP (PP+). Our primary endpoint was the influence of PP+ on overall survival (OS), while the progression-free survival (PFS) and locoregional PFS (LRPFS) constituted the secondary endpoints.
Results
The PP+ was detected in 29 (27.9%): nine (8.7%), seven (6.7%), and 13 (12.5%) in the primary tumor alone, primary tumor plus neck nodes, and neck nodes alone, respectively. Because the PP+ cases were small by count per location, all comparative analyses were performed according to overall PP+/ PP– status instead of per detected site. At a median follow-up of 67.8 months (range, 9 to 130 months), the median survival times were not reached (NR) for the entire population, while 5-year OS, LRPFS, and PFS rates were 73.3%, 68.2%, and 63.4%, respectively. Comparatively the PP+ patients exhibited significantly poorer median OS (49.8 months vs. NR, P<0.001), LRPFS (40.7 months vs. NR, P=0.001), and PFS (31.8 months vs. NR, P=0.002) durations than their PP– counterparts. Furthermore, the PP+ retained its independent prognostic significance in multivariate analysis (P<0.001).
Concurrent chemoradiotherapy (C-CRT) has long been the standard treatment for locoregionally advanced nasopharyngeal cancers (NPCs) [1,2]. Multidisciplinary decision to guide the treatment for NPC is mainly based on T, N, and M definitions of tumor-node-metastasis (TNM) staging [3], while outcomes might significantly vary among homogeneously treated NPC patients in identical stages; for the most part due to ignorance of various biochemical, clinical, nutritional, molecular, and viral factors [4,5].
18F-fluorodeoxyglucose positron emission tomography (FDGPET) has been integrated into the staging of NPCs, predominantly for its precision to preclude possible distant metastasis (DM). Consequently, various FDG parameters have been proposed as prognostic factors in years [6,7]. The maximal standardized uptake value (SUVmax), the metabolic tumor volume, and the total lesion glycolysis (TLG) are the most commonly studied metabolic and volumetric parameters for their possible roles in the prediction of patients’ prognosis in several tumors [8-10]. The FDG-PET defined photopenia (PP) in the primary tumor, a predictor of tumor ischemia-necrosis, was previously tested as another parameter for its influence on the survival results of non-small-cell lung cancer patients in a prospective Trans-Tasman observational study treated with radiotherapy (RT) or C-CRT [11]. The hypothesis was based on the survival correlation of tumor necrosis in photopenic (PP+) regions containing hypoxic but possibly viable and relatively radioresistant cancer cells in comparison to fully oxygenated parts of the tumor without PP (PP–). Though the impact of hypoxia on the survival of NPCs has been studied with various tracers [12-14], yet, PP in FDG-PET has never been defined as a prognostic factor to date. Thusly, we hypothesized that PP+ NPC patients may have worse outcomes in comparison to their PP– counterparts after definitive C-CRT.
We have retrospectively searched our institutional database to identify the NPC patients treated with C-CRT between January 2007 and December 2015. The key eligibility criteria were: (1) aged 18 to 80 years, (2) Karnofsky performance score ≥70, (3) histologically proven squamous cell carcinoma (SCC), (4) clinical radiological proof of T1-4, N0-3, M0 according to the TNM staging system (7th ed), (5) no prior RT/chemotherapy history, (6) received platinum-based C-CRT, (7) available FDG-PET-CT scans, (8) no radiological evidence of brain metastasis, and (9) available RT and chemotherapy charts, pre-C-CRT complete blood count and biochemistry tests, and pre-C-CRT and followup head and neck clinical and radiological examinations.
Each patient either themselves or via legally authorized representatives provided written informed consent before treatment for collection and analysis of blood samples, pathologic specimens, and publication of their outcomes as indicated by our institutional standards. The Institutional Review Board of Baskent University Medical Faculty approved the study design before the collection of any patient data.
As previously described elsewhere [5], all patients received definitive C-CRT, via three-dimensional conformal RT between January 2007 to June 2011 and intensity-modulated RT (IMRT), afterwards. All RT was prescribed in a daily fractionation basis: 5 day/wk, for 7 weeks. Concurrent chemotherapy included cisplatin 75–80 mg/m2 (every 3 weeks), and adjuvant chemotherapy involved two cycles of cisplatin-based regimens (every 3 weeks).
The PP was blindly evaluated from pretreatment FDG-PET scans by a nuclear medicine physician (NT) and a radiation oncologist (ET) experienced on head and neck cancers and was defined as an internal volume within the primary and/or nodal tumor volume with an SUV less than 40% of the SUVmax according to the Trans-Tasman observational study reported by Ashley Cox et al. (Fig. 1) [11]. Primary tumors and involved nodes were then classified as PP+ or PP–. Contrasts were discussed by the two authors on a case by case premise and the achieved accord was recorded as the final consensus decision on PP+/PP– status, whenever indicated.
Treatment response was documented regularly every 3 months for the first 2 years, every 6 months between the 3rd to 5th years, and annually (or more often) thenceforth. Each evaluation covered detailed endoscopic examinations for the index NPC and neck regions in order to ascertain any local/regional recurrences. Based on our clinical standards, the treatment response was assessed prospectively for all patients despite of the retrospective study design. First restaging PET-CT scans were obtained at the 90-day follow-up visit and scored per to the European Organisation for Research and Treatment of Cancer-1999 guidelines (the PET Response Criteria in Solid Tumors after 2009). The PET-CT was replaced by the head and neck CT and/ or magnetic resonance imaging (MRI) scans after confirmation of a metabolic complete response.
Primary endpoint was the association between PP+ in pretreatment FDG PET scans and overall survival (OS): interval between the onset of C-CRT and death or last visit. Secondary endpoints included the associations between PP+ and progression-free survival (PFS) or locoregional PFS (LRPFS): the interval between the onset of C-CRT and any type of disease progression or last visit/death (for PFS), or progression/recurrence at the nasopharynx and/or ipsilateral/contralateral neck or last visit/death (for LRPFS), respectively.
Categorical and continuous variables were described by frequency distributions and means, medians, and ranges as indicated. Chi-square tests, Student t-tests, Pearson’s exact test, or Spearman correlations were utilized to compare frequency distributions among different groups and their correlations, as appropriate. Frequency distributions were utilized for categorical variables, while means, medians, and ranges were used to describe continuous variables. The influence of different variables on OS, LRPFS, and PFS was evaluated with Kaplan-Meier estimates and log-rank tests. The multivariate analysis incorporated only the factors exhibiting significance in univariate analysis and tested with utilizing the Cox proportional hazards model. A two-sided P-value <0.05 was considered significant.
Our database search revealed 104 NPC patients eligible for analyses. Patients were grouped into two per PP status: PP– (n=75, 72.1%) and PP+ (n=29, 27.9%) respectively based on the pre–C-CRT PET-CT findings. Of the 29 PP+ patients; nine (8.7%), seven (6.7%), and 13 (12.5%) had PP in the primary tumor alone, primary tumor plus neck nodes, and neck nodes alone, respectively. However, all analyses were performed on a “PP– versus PP+” basis regardless of the location due to the limited number of PP+ patients at each site. Baseline patients’ demographics were as depicted in Table 1, with no notable differences between the two groups. Of note, contrasts were discussed by the two authors for PP+/PP– status in only two of 104 cases (1.9%) demonstrating a 98.1% initial agreement between the two authors.
At a median follow-up of 67.8 months (range, 9 to 130 months), 74 patients (71.2%) were still alive and 73 (70.2%) of them were free of any disease progression. Overall DM was the commonest relapse pattern (n=26, 25.0%), while 11 patients (11.5%) experienced local/regional recurrences during the follow-up period. Neither of the median OS, LRPFS, and PFS durations was achieved for the whole study population during this final analysis, while the 5- and 10-year survival rates were 73.3% and 56.0% for OS, 68.2% and 53.2% for LRPFS, and 63.4% and 48.0% for PFS, respectively.
Comparative analyses between the two PP groups revealed that either of the median OS (not reached [NR] vs. 49.8 months, P<0.001), LRPFS (NR vs. 40.7 months, P<0.001), and PFS (NR vs. 31.8 months, P<0.001) was significantly superior in the PP– group than its PP+ counterpart, respectively (Fig. 2). Similarly the 5- and 10-year survival rates were also significantly better in the PP– group (Table 2). Additionally, the 5- and 10-year actuarial locoregional failure (LRF; P=0.004 and P<0.001) and DM (P=0.002 and P<0.001) rates were significantly lower in the PP– group (Table 2). We likewise performed comparative analyses among the 29 PP+ patients which revealed no significant difference among the three PP+ cohorts with regards to the OS (P=0.79), LRPFS (P=0.57), OS (P=0.001), LRF (P=0.68), and DM (P=0.39) outcomes, respectively.
In univariate analysis, besides the PP status (PP– vs. PP+), the T-stage (1–2 vs. 3–4), N-stage (0–1 vs. 2– 3), and TNM stages (2– 3 vs. 4A–B) were identified to be the factors demonstrating significant link with superior PFS, LRPFS, and OS outcomes (Table 3). The results of multivariate analysis restricted to these factors confirmed the independent prognostic significance of each variable on all survival endpoints.
We also searched for the presence of a significant link with the respective percentage PP size [(maximum diameter of PP/ maximum diameter of tumor)×100] and percentage PP volume [(PP volume/tumor volume)×100] relative to the tumor size and volume which may interact with the survival outcomes. However, receiving operator characteristic curve analysis did not reveal any critical cutoffs that may dichotomize patients into two PP+ groups for further comparative analyses.
Outcomes of our single-center cohort analysis exhibited that the PP+ shown on pretreatment FDG-PET was a strong and independent associate of poorer survival outcomes in NPC patients who underwent C-CRT. Hypothetically, these results vocalize the requirement for the fortification of the local and systemic treatments in PP+ patients to defeat the metabolically evident ischemia, and therefore, the resistance to C-CRT.
The central tumor or lymph node PP on PET, or cavitation on CT, is recognized as a consequence of tumor necrosis caused by chronic ischemia/hypoxia. Heterogeneously dispersed hypoxic regions are verifiable in more than half of all solid tumors irrespective of the size and histology, although hypoxia is a more frequent feature of large tumors and SCC histology. The hypoxia concern in head and neck cancers has been raised for a long time to decide its significance and to indicate strategies to defeat for its obvious negative influence [15-17]. Earliest endeavors at distinguishing tumor hypoxia focused on the vascular O2 supply by concentrating on oxyhemoglobin saturation or tumor perfusion (MRI, CT, PET) [18], while subsequent investigations utilized endogenous markers either individually or in gene signatures to identify hypoxia, such as hypoxia-inducible factor-1α, carbonic anhydrase IX, glucose transporters 1 and 3, and osteopontin [19,20]. Later, measurements with exogenous markers have become popular, and the activation of nitroheterocyclic drugs by cellular nitroreductases provided an assay to estimate the degree of cellular hypoxia in vivo: invasive ones measuring pO2 such as polarographic electrodes, or immunohistological analysis of biopsy specimens with etanidazole; noninvasive ones such as PET, single-photon emission CT, or MRI with radioactively labeled nitroimidazoles or PET imaging with copper II-diacetyl-bis N4-methylthiosemicarbazone [14]. Unfortunately, aside from the invasive techniques which provide the direct estimates of tumor oxygenation, none of the above approaches are perfect: hypoxia isn’t related with the vasculature alone; endogenous markers are not explicit and can be upregulated by stress conditions even under normoxic settings, and exogenous markers are nonreproducible. Although our study was designed to uniquely focus on the PP+/PP– status of the index NPC and involved lymph nodes, yet considering the recommended 55– 75-minute interval between the FDG administration and the start of the PET scanning, we rationally assume that the hypoxia-associated PP in FDG-PET observed here might most possibly be related with chronic hypoxia, as opposed to only a glimpse of acute hypoxia.
Previously, necrosis (PP on FDG-PET) has been reported in the form of cervical nodal necrosis (CNN) with an incidence ranging from 20% to 44.1% in NPC patients with neck involve ment at pretreatment MRI [21], with which our PP+ incidence of 27.9% appears to be in good agreement. We acknowledge the difficulties in the comparison of studies with different methodologies; nonetheless, our results displayed here appear to be in good accordance with the accessible NPC literature concerning the survival endpoints. To begin with, Tang et al. [22] evaluated the influence of MRI-detected CNN in metastatic retropharyngeal lymph nodes and concluded that CNN was a significant independent prognostic factor for distant metastasis-free survival (DMFS) and LRRFS in NPCs treated with IMRT. Likewise, Lan et al. [21] documented the CNN as an independent prognostic factor in a retrospective cohort of 1,302 newly diagnosed NPCs, with significantly superior 5-year OS (78.8% vs. 91.8%, P<0.001), PFS (78.2% vs. 91.2%, P<0.001), regional PFS (78.6% vs. 91.8%, P<0.001), and DMFS (78.4% vs. 91.6%, P<0.001) favoring the group without CNN [21]. Furthermore, the authors revealed poorer survival rates in patients with CNN similar to their counterparts with a higher nodal stage without CNN and proposed the incorporation of CNN into the American Joint Committee on Cancer staging framework for NPC. Zhang et al. [23], in another study incorporating 1,423 NPC patients with cervical nodal metastasis analyzed the prognostic value of CNN distinguished on pretreatment MRI. The CNN was quantified as grade 0 with no, grade 1 with ≤33%, and grade 2 with >33% hypodense zones. Once more, affirming the aforementioned studies, the authors reported that all survival end-points were significantly superior in the grade 0 CNN group. Although our study differs from these studies due to examination of primary tumor and nodal PP+ status altogether, yet, our significantly inferior 5- and 10-year OS, LRPFS, PFS, LRF, and DM rates in the PP+ group appear to confirm the PP+ as a relevant poor prognostic factor for NPC patients treated with RT or C-CRT, likewise the above-mentioned studies.
Another important contribution of our present research to the NPC literature was the exhibition of notably higher 5- and 10-year LRF and DM rates in the PP+ gathering. Though it is very hard to allocate these discoveries to explicit causes with our current knowledge, yet some reasonable comments can be made. Other than being a useful indicator of extracapsular nodal spread, tumor necrosis or PP, a marker of intratumoral hypoxia, is likewise a settled factor that induces or contributes to resistance to RT and chemotherapy [14]. Amassing clinical proof has clearly shown that the poor O2 status was unequivocally connected with significantly inferior response to RT with resultant diminished locoregional control rates and survival times in head and neck cancers including the NPCs [14]. Supporting the “O2 fixation hypothesis” which proposes that the radiation-induced DNA lesions will less likely be permanent in the hypoxic regions, Nordsmark and Overgaard [24] showed that the proportion of pO2 values <2.5 mmHg was independently associated with LRF rates [24]. Similarly, we recently demonstrated that pre–C-CRT hemoglobin <11.0 g/dL (another surrogate marker of hypoxia), had a stronger prognostic worth than the anemia status respecting the LRF rates and LRPFS durations for NPC patients [5]. In another study, Lin et al. [18] examined the pretreatment CT and PET/CT of 91 pharyngeal cancers treated with definitive RT/C-CRT, and distinguished the CNN (hazard ratio [HR], 10.99) and N-TLG40% (40% of the maximal uptake of nodal TLG) ≥38 g (HR, 2.63) as the two independent adverse risk factors for nodal relapse-free survival. We believe that our affirmative higher 5-year LRF (24.1% vs. 8.0%, P<0.001) and lesser LRPFS (39.8 vs. 79.0 months, P<0.001) rates in PP+ group observed here lend further support on previous studies proposing chemo- and/or radioresistance in hypoxic tumors as a significant contributor of treatment failures.
As a consequence of enhanced locoregional control rates with refined C-CRT regimes, the DM turned into the leading failure pattern for NPCs, with an estimated rate of over 20% [25]. The exact mechanisms underlying the aggressiveness of hypoxic NPC phenotypes and related high DM rates are not fully elucidated. Nevertheless, other than the enhancement of the metastatic potential, hypoxia has also been proposed to prompt poor drug delivery to the primary or metastatic disease foci, which renders it difficult to eradicate the nonquantifiable but still via ble microscopic DMs. Affirming this proposal, growth and cellular invasiveness of the NPC cells have been clearly shown to be inhibited by the molecules targeting tumor hypoxia [26]. For instance, higher levels of hypoxia-induced lysyl oxidase (LOX), an extracellular matrix protein with many functions including metastasis enhancement, was found to be associated with lower rates of DMFS and OS in HNCs, while LOX inhibition was shown to reduce hypoxia-induced tumor invasion and metastasis in breast and cervical cancers, which may also be relevant for NPCs [27]. Similarly, hypoxia-induced upregulation of serine protease inhibitor protein (SERPINE-1), a target gene of HIF1α, was shown to be induced by hypoxia in NPCs [27]. Furthermore, the hypoxia marker HIF-1α and its downstream target gene VEGF also facilitate NPC metastasis, and their coexpression was shown to be significantly correlated with higher DM, local recurrence, and poor patient survival in NPCs [28]. Confirming the importance of inhibition of hypoxia, the selective hypoxia inhibitor tirapazamine was demonstrated to effectively reduce the levels of HIF-1α and VEGF in vitro [26]. Therefore, the accessible basic knowledge has exhibited that the hypoxia and DM were legitimately correlated in many tumor types, including the NPCs. Clinically; our present significantly higher actuarial 5-year DM rate (34.5% vs. 16.0%, P=0.002) in the PP+ cohort appears to confirm this preclinical evidence. Clinically, underscoring the prognostic importance of hypoxia in NPC patients, recently it was reported that the pre-C-CRT hemoglobin <11.0 g/dL had a stronger prognostic worth than the anemia status with regards to the LRPFS (P=0.004), PFS (P=0.004), and OS (P=0.004) [5]. Furthermore, the DMFS was shown to be significantly affected between the all CNN grades in the Zhang’s aforementioned study [23], bringing the contention that even minimum hypoxic necrosis (grade 1) seems to be not clinically different from more hypoxic necrosis such as grade 2 in terms of DMFS, as an “all or none phenomenon”.
The research displayed here was strengthened by some factors. To begin with, we consistently treated all patients in a comparable design regarding the C-CRT and adjuvant chemotherapy conventions, except for the timely replacement of three-dimensional-CRT by IMRT. Moreover, besides uniform use of PET-CT for disease staging and RT planning, our single-center cohort who had all scans performed in the same institution under supervision of the same team strengthened the comparability and legitimacy of our results, as the homogeneity and PET imaging thresholds in staging FDG-PET/CT imaging processes are of proven clinical importance. However, our investigation was also subject to numerous drawbacks: first, it was a single-institutional retrospective analysis with a relatively small cohort size. Second, although we examined the comparative effects of PP in the primary tumors, lymph nodes on the survival outcomes, yet the observation of no significant differences in intergroup comparisons may not reflect the true individual impact of PP+ location considering that there were only a total of 29 PP+ patients in all three groups. Therefore, this particular issue may be an interesting research area for future research in large scale studies with sufficient discriminatory statistical power. Third, nonattendance of the other established hypoxia markers such as the HIF-1, HIF-2, CA-IX, GLUT-1, GLUT-3, and osteopontin; absence of direct intratumoral O2 measurements and more specific hypoxia tracers during the PET scanning (inaccessible in our country) may have limited our ability to achieve more convincing remarks on this critically important subject. And lastly, differences among the salvage therapies in patients who experienced recurrences might also have unpredictably altered our results in favor of one group. Therefore, we emphatically recommend that present observations reported here ought to be acknowledged as just the results of a first endeavor addressing the prognostic significance of PP on pre–C-CRT PET examines of NPC patients, as opposed to being indisputable remarks on the subject until the outcomes of well-designed future studies addressing the above-mentioned particular restrictions become accessible.
We demonstrated that the hypoxia marker PP+ on pretreatment FDG-PET was a strong independent indicator of poor LRPFS, PFS, and resultant OS outcomes even in the absence of specific genetic signatures and metabolic/biochemical hypoxia tracers. Moreover, because the impact of PP+ on the LRPFS and PFS outcomes (P<0.001 for each) was comparable, our results in like manner suggest the PP+ as a common marker of chemo- and/or radioresistance and an aggressive NPC phenotype with high metastatic potential.
▪ Impact of pretreatment 18F-fluorodeoxyglucose positron emission tomography photopenia (PP) on the results of nasopharyngeal cancers (NPCs) was analyzed.
▪ PP was incident in 29 (27.9%) of 104 NPC patients.
▪ PP was independently associated with poor local and distant control rates and survival times.
▪ PP presence appeared to indicate a highly metastatic NPC phenotype that is chemo- and radio-resistant.
REFERENCES
1. Lin JC, Jan JS, Hsu CY, Liang WM, Jiang RS, Wang WY. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol. 2003; Feb. 21(4):631–7.

2. Chan AT, Teo PM, Ngan RK, Leung TW, Lau WH, Zee B, et al. Concurrent chemotherapy-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: progression-free survival analysis of a phase III randomized trial. J Clin Oncol. 2002; Apr. 20(8):2038–44.

3. Lydiatt WM, Patel SG, O’Sullivan B, Brandwein MS, Ridge JA, Migliacci JC, et al. Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017; Mar. 67(2):122–37.

4. Li J, Chen S, Peng S, Liu Y, Xing S, He X, et al. Prognostic nomogram for patients with nasopharyngeal carcinoma incorporating hematological biomarkers and clinical characteristics. Int J Biol Sci. 2018; Apr. 14(5):549–56.

5. Topkan E, Ekici NY, Ozdemir Y, Besen AA, Yildirim BA, Mertsoylu H, et al. Baseline hemoglobin <11.0 g/dL has stronger prognostic value than anemia status in nasopharynx cancers treated with chemoradiotherapy. Int J Biol Markers. 2019; Jun. 34(2):139–47.
6. Chung MK, Jeong HS, Park SG, Jang JY, Son YI, Choi JY, et al. Metabolic tumor volume of [18F]-fluorodeoxyglucose positron emission tomography/computed tomography predicts short-term outcome to radiotherapy with or without chemotherapy in pharyngeal cancer. Clin Cancer Res. 2009; Sep. 15(18):5861–8.
7. Paidpally V, Chirindel A, Chung CH, Richmon J, Koch W, Quon H, et al. FDG volumetric parameters and survival outcomes after definitive chemoradiotherapy in patients with recurrent head and neck squamous cell carcinoma. AJR Am J Roentgenol. 2014; Aug. 203(2):W139–45.

8. Nakamura K, Joja I, Kodama J, Hongo A, Hiramatsu Y. Measurement of SUVmax plus ADCmin of the primary tumour is a predictor of prognosis in patients with cervical cancer. Eur J Nucl Med Mol Imaging. 2012; Feb. 39(2):283–90.
9. Castelli J, Depeursinge A, de Bari B, Devillers A, de Crevoisier R, Bourhis J, et al. Metabolic tumor volume and total lesion glycolysis in oropharyngeal cancer treated with definitive radiotherapy: which threshold is the best predictor of local control. Clin Nucl Med. 2017; Jun. 42(6):e281–5.
10. Choi WR, Oh JS, Roh JL, Kim JS, Oh I, Choi SH, et al. Metabolic tumor volume and total lesion glycolysis predict tumor progression and survival after salvage surgery for recurrent oral cavity squamous cell carcinoma. Head Neck. 2019; Jun. 41(6):1846–53.

11. Ashley Cox R, Akhurst T, Bressel M, MacManus M, Ball D. Survival and central photopenia detected by fluorine-18 fluoro-deoxy-glucose positron emission tomography (FDG-PET) in patients with locoregional non-small cell lung cancer treated with radiotherapy. Radiother Oncol. 2017; Jul. 124(1):25–30.

12. Xie W, Liu L, He H, Yang K. Prognostic value of hypoxia-inducible factor-1 alpha in nasopharyngeal carcinoma: a meta-analysis. Int J Biol Markers. 2018; Nov. 33(4):447–54.

13. Yip C, Cook GJ, Wee J, Fong KW, Tan T, Goh V. Clinical significance of hypoxia in nasopharyngeal carcinoma with a focus on existing and novel hypoxia molecular imaging. Chin Clin Oncol. 2016; Apr. 5(2):24.

14. Hong B, Lui VW, Hashiguchi M, Hui EP, Chan AT. Targeting tumor hypoxia in nasopharyngeal carcinoma. Head Neck. 2013; Jan. 35(1):133–45.

15. Bredell MG, Ernst J, El-Kochairi I, Dahlem Y, Ikenberg K, Schumann DM. Current relevance of hypoxia in head and neck cancer. Onco target. 2016; Aug. 7(31):50781–804.

16. Al-Zoughbi W, Huang J, Paramasivan GS, Till H, Pichler M, GuertlLackner B, et al. Tumor macroenvironment and metabolism. Semin Oncol. 2014; Apr. 41(2):281–95.
17. Horsman MR, Mortensen LS, Petersen JB, Busk M, Overgaard J. Imaging hypoxia to improve radiotherapy outcome. Nat Rev Clin Oncol. 2012; Dec. 9(12):674–87.

18. Lin YC, Chen SW, Hsieh TC, Yen KY, Yang SN, Wang YC, et al. Risk stratification of metastatic neck nodes by CT and PET in patients with head and neck cancer receiving definitive radiotherapy. J Nucl Med. 2015; Feb. 56(2):183–9.

19. Koukourakis MI, Bentzen SM, Giatromanolaki A, Wilson GD, Daley FM, Saunders MI, et al. Endogenous markers of two separate hypoxia response pathways (hypoxia inducible factor 2 alpha and carbonic anhydrase 9) are associated with radiotherapy failure in head and neck cancer patients recruited in the CHART randomized trial. J Clin Oncol. 2006; Feb. 24(5):727–35.

20. Rademakers SE, Lok J, van der Kogel AJ, Bussink J, Kaanders JH. Metabolic markers in relation to hypoxia; staining patterns and colocalization of pimonidazole, HIF-1α, CAIX, LDH-5, GLUT-1, MCT1 and MCT4. BMC Cancer. 2011; May. 11:167.

21. Lan M, Huang Y, Chen CY, Han F, Wu SX, Tian L, et al. Prognostic value of cervical nodal necrosis in nasopharyngeal carcinoma: analysis of 1800 patients with positive cervical nodal metastasis at MR imaging. Radiology. 2015; Aug. 276(2):536–44.

22. Tang L, Li L, Mao Y, Liu L, Liang S, Chen Y, et al. Retropharyngeal lymph node metastasis in nasopharyngeal carcinoma detected by magnetic resonance imaging: prognostic value and staging categories. Cancer. 2008; Jul. 113(2):347–54.
23. Zhang LL, Li JX, Zhou GQ, Tang LL, Ma J, Lin AH, et al. Influence of cervical node necrosis of different grades on the prognosis of nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapy. J Cancer. 2017; Mar. 8(6):959–66.

24. Nordsmark M, Overgaard J. Tumor hypoxia is independent of hemoglobin and prognostic for loco-regional tumor control after primary radiotherapy in advanced head and neck cancer. Acta Oncol. 2004; 43(4):396–403.

25. Lee AW, Ng WT, Chan LL, Hung WM, Chan CC, Sze HC, et al. Evolution of treatment for nasopharyngeal cancer: success and setback in the intensity-modulated radiotherapy era. Radiother Oncol. 2014; Mar. 110(3):377–84.
26. Hong B, Lui VW, Hui EP, Ng MH, Cheng SH, Sung FL, et al. Hypoxia-targeting by tirapazamine (TPZ) induces preferential growth inhibition of nasopharyngeal carcinoma cells with Chk1/2 activation. Invest New Drugs. 2011; Jun. 29(3):401–10.

27. Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006; Apr. 440(7088):1222–6.

28. Xueguan L, Xiaoshen W, Yongsheng Z, Chaosu H, Chunying S, Yan F. Hypoxia inducible factor-1 alpha and vascular endothelial growth factor expression are associated with a poor prognosis in patients with nasopharyngeal carcinoma receiving radiotherapy with carbogen and nicotinamide. Clin Oncol (R Coll Radiol). 2008; Oct. 20(8):606–12.
Fig. 1.
Demonstration of photopenia on fluorodeoxyglucose positron emission tomography-computed tomography scans. (A) Primary tumor (arrow). (B) Neck node (arrow).
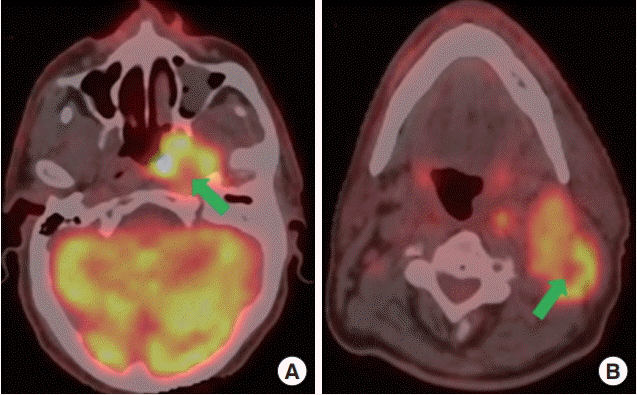
Fig. 2.
Kaplan-Meier survival estimates according to photopenia (PP) status. (A) Overall survival. (B) Locoregional progression-free survival. (C) Progression-free survival.
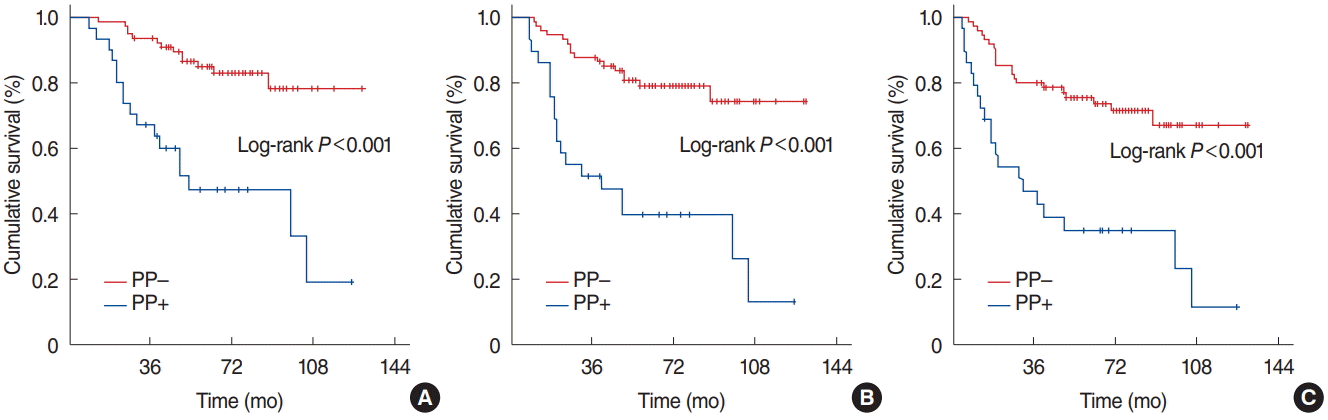
Table 1.
Baseline characteristics of 104 patients with locoregionally advanced nasopharyngeal carcinoma
Table 2.
Survival outcomes according to PP status
Table 3.
Outcomes of uni- and multivariate analysis




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download