Abstract
Objectives
This study aimed to evaluate the clinical characteristics of maxillary sinus fungus ball (MFB) to increase the preoperative diagnostic accuracy.
Methods
A retrospective review of 247 patients who underwent endoscopic sinus surgery for unilateral maxillary sinusitis from January 2015 to December 2017 at a single institution was performed. Patients with pathologically proven MFB were compared to those with unilateral chronic maxillary sinusitis (CMS). Patient demographics and computed tomography (CT) findings were evaluated. The CT features were categorized as intralesional hyperdensity (calcification), the irregular lobulated protruding lesion (fuzzy appearance), maxillary sinus full haziness without mass effect, maxillary sinus full haziness with mass effect, and others. A regression tree analysis was performed.
Results
In total, 247 patients were analyzed; among them, 179 (72.5%) had MFB and 68 (27.5%) had CMS. MFB showed predominance in older individuals. Among the radiological features, intralesional hyperdensity was most commonly associated with MFB. The presence of a fuzzy appearance or full opacity with mass effect was also associated with MFB. The highest area under the curve was noted with the regression tree analysis based on the model, which included the presence of intralesional hyperdensity, demographic data (age), and presence of fuzzy appearance or maxillary sinus full haziness with mass effect in case of absence of intralesional hyperdensity (0.904).
Sinonasal fungus ball is the most common form of fungal rhinosinusitis that usually occurs in adults with a normal immune system [1-3]; the most commonly involved site is a solitary maxillary sinus [2]. Although the pathogenesis is still unclear, previous studies indicate the role of sex and age as sinonasal fungus ball is predominant in women and those of older age [2-4]. Patients with sinonasal fungus ball usually present with nasal obstruction, facial pain, postnasal drip and purulent rhinorrhea; however, they are sometimes asymptomatic [1,3]. Fungus ball may lead to obstruction of the natural ostium and can cause secondary chronic rhinosinusitis (CRS). The initial treatment of choice for CRS is medical therapy, whereas for the fungus ball, surgical intervention would be preferred [2]. Although it is difficult to differentiate CRS from fungus ball, especially if it is unilateral and without polyposis, it is important for determination of the optimal treatment. Previous studies have reported well verified maxillary sinus fungus ball (MFB) computed tomography (CT) features including intralesional hyperdensity (presence of calcified lesions), irregular surface of the material, complete opacification in sinus with or without expansion sinus and absence of air fluid level [1,5-7]. Among them, presence of intralesional hyperdensity is known to be a highly predictive radiological parameter for fungus ball. The diagnostic criteria based on CT findings have been clearly defined by deShazo et al. [5] and are highly specific (99%) [8]. Previous studies found that 50%–83% of fungus balls present with intralesional hyperdensity on CT imaging; hence, the sensitivity was found to be only about 62% [1,3,9,10]. A significant proportion of patients may not present with these lesions; in such patients, diagnosis is difficult. The purpose of this study was to evaluate the clinical characteristics of MFB and to increase the preoperative diagnostic accuracy based on these characteristics.
This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (IRB No. B-1903-528-103).
A retrospective medical review of patients who underwent unilateral endoscopic sinus surgery (ESS) due to medically intractable maxillary sinusitis from January 2015 to December 2017 at the Seoul National University Bundang Hospital was performed. The diagnosis was based on histopathological examination of surgical materials from the maxillary sinus and it was double checked with CT scan and operation record. Lesions primarily located on sinuses other than maxillary sinus were excluded. MFB was diagnosed based on the presence of dense material in the maxillary sinus and dichotomous branching of fungal hyphae on Gomori methenamine silver stain and periodic acid Schiff stain. Absence of such findings led to the lesion being diagnosed as chronic maxillary sinusitis (CMS); however, cases with CMS presumably associated with odontogenic causes, observed as the presence of periapical lucency or bony dehiscence over the tooth root on CT scan [11]. Moreover, CRS combined with other pathologies such as postoperative maxillary cyst, inverted papilloma, malignant tumor, or antrochoanal polyps were excluded.
Demographic characteristics, including age and sex, presence of diabetes mellitus (DM), and CT findings of the maxillary lesions were evaluated. Non-contrast CT scan was performed on a single-source multi-detector CT with 256 channels (iCT; Philips, Amsterdam, the Netherlands). Imaging parameters for the scanning were as follows: 120 kVP; 200 mAs; axial scan mode; pitch, 0.39; detector collimation, 64×0.625 mm; gantry rotation time, 0.5 seconds; slice thickness, 0.9 mm. Images were obtained from the upper margin of the frontal sinus to the lower margin of the mandible. The acquired raw data were then reconstructed with 2-mm section thickness. The maxillary sinus lesion was evaluated on three planes (axial, sagittal and, coronal) reconstructed images, using window width of 2,000 and window level of 0 for the bone and calcification, and window width of 300 and window level of 30 for the soft-tissue lesion. The CT features were categorized as (1) presence of intralesional hyperdensity (calcification), (2) irregular lobulated protruding lesion (fuzzy appearance), (3) maxillary sinus full haziness without mass effect, (4) maxillary sinus full haziness with mass effect, and, (5) others, such as smooth (wider than tall) protruding lesion, rather than fuzzy appearance, suggesting mucosal thickening or fluid collection (Fig. 1). Repeated selection was accepted between intralesional hyperdensity and other features. Lesions with mass effect and expansile nature were represented as widening of the ostiomeatal unit or thinning of the wall of the involved maxillary sinus. Demographic and radiological features of patients with MFB were compared with those of patients with unilateral CMS.
Cross-table analysis with chi-square test or Fisher’s exact test was used for categorical variables, whereas independent t-test was used for continuous parametric variables. Firth logistic regression analysis was performed for risk evaluation of combination of factors. Statistical significance was set at P-value <0.05. To find an optimal algorithm to differentiate the two pathologies, a classification tree based on classification and regression trees was generated using the input variables that were used for comparison between MFB and CMS. To establish and validate classifiers, 10-fold cross-validation was used, and receiver operating characteristic curve was used to assess the predictive value of clinical parameters. Classification tree analysis was performed by WEKA [12]. Other statistical analyses were performed using IBM SPSS 22.0 (IBM Corp., Armonk, NY, USA). Results are presented as means and standard deviations.
First, 728 patients who underwent unilateral ESS were reviewed. After exclusion; lesions primarily located in sinuses other than maxillary sinus (n=72), CRS combined with other pathologies (n=403), CMS presumably associated with odontogenic causes (n=6), 247 patients were analyzed finally (Fig. 2).
This study included 179 patients with MFB and 68 patients with CMS. The mean age of MFB patients was significantly higher than that of CMS patients (61.1±13.4 years vs. 48.2±17.9 years, P<0.001). There was also a female predominance among patients with MFB compared to those with CMS (male to female ratio, 0.47 vs. 1.19; P=0.002). However, there was no significant difference in the presence of comorbid DM (5.6% and 2.9% in patients with MFB and CMS, respectively, P=0.520) (Table 1).
Intralesional hyperdensity was noted in 134 (74.9%) of 179 cases of MFB and in none (0%) of the cases of CMS (P<0.001). Among other features, fuzzy appearance was also associated with MFB; this was observed in 69.1% (55/179) of MFB cases and only 7.4% (5/68) of CMS cases (P<0.001). Despite the fact that there was no significant difference in the presence of full haziness in the maxillary sinus without mass effect in cases of MFB compared to those of CMS (11.2% vs. 14.7%, respectively, P=0.513), the presence of mass effect was significantly more associated with cases of MFB (33.5% vs. 8.8%, respectively, P<0.001). Other features, such as smooth protruding lesion suggesting mucosal thickening or fluid collection, were more commonly associated with CMS patients than MFB patients (67.6% vs. 25.1%, respectively; P<0.001). The radiologic characteristics are summarized in Table 2.
Factors that were significant in univariate analysis (i.e., age, sex, and radiological features including intralesional hyperdensity, fuzzy appearance, maxillary sinus full haziness with mass effect, and others) were than reviewed with firth logistic regression analysis. According to our data, presence of intralesional hyperdensity (odds ratio [OR], 295.567; 95% confidence interval [CI], 39.226 to 37,922.87; P<0.001), fuzzy appearance (OR, 9.519; 95% CI, 3.228 to 32.078; P<0.001), maxillary sinus full haziness with mass effect (OR, 7.140; 95% CI, 2.476 to 22.781; P<0.001), and increasing age (OR, 1.028; 95% CI, 1.002 to 1.056; P=0.032) were significantly associated with MFB. MFB was predominant in female, however not significant according to logistic regression analysis (OR, 1.008; 95% CI, 0.415 to 2.451; P=0.985) (Table 3).
Three models (model 1 to 3) were validated from the regression tree analysis. Model 1 only involved intralesional hyperdensity as a variable; model 2 added demographic data; and model 3 included intralesional hyperdensity and demographic data, as well as other radiological features. The area under the curve of models 1 to 3 based on the decision tree model were 0.853, 0.880, 0.904, respectively. This suggests the highest predictive value in model 3 (Fig. 3). The accuracy of each model was 81.781%, 79.757%, 88.664%. Other values are described in Table 4.
Fungus ball in paranasal sinuses, once considered to be a rare disease, is recently gaining clinical attention because of the dramatic increase in its incidence over the past two decades in Korea [4,13]. With regard to treatment, it should be surgically removed with adequate drainage of the involved sinuses. However, in cases of unilateral CRS from other causes, medical therapy could be initiated as a first-line treatment. Further, there is always possibility of the fungus ball progressing to an invasive form, which has a significant worse disease course even after optimal treatment [14]. Therefore, early diagnosis and proper treatment of sinonasal fungus ball is of utmost importance. However, a definitive diagnosis, which is the pathologic confirmation of the fungal hyphae or mycotic colonization [3], can only be made after surgery.
Among the current available diagnostic tools, CT is the optimum for detecting fungus ball preoperatively, and the most significant finding on the CT scan is the presence of intralesional hyperdensity [5]. According to previous studies, the prevalence of intralesional hyperdensity lesion on the CT scan was 50%– 83% [1,3,9,10]. In the current study, despite the high specificity (1.0), the sensitivity for intralesional hyperdensity in MFB was rather low (0.749), suggesting that with only intralesional hyperdensity, there is a considerable risk of false negative results. Therefore, this study aimed to find other clinical factors that may increase the overall diagnostic accuracy.
Presence of intralesional hyperdensity detected on a CT scan suggests the presence of metal metabolites from the fungal organism [15]. Aspergillus, which comprises most of the organism related to paranasal sinus fungus ball [16], stores metal ions such as zinc in the intracellular vacuole storage system to keep the homeostasis of the zinc ion which is known to be an essential nutrient for the organism [17]. However, in cases of zinc deprivation, down regulation of the genes related to the storage of zinc occurs [18] and under these conditions, zinc concentration in the vacuole decreases and therefore may be reflected by the absence of intralesional hyperdensity on the CT scan.
Our study demonstrated the importance of radiological features, apart from intralesional hyperdensity, in the diagnosis of MFB. Among other radiological features, fuzzy appearance and maxillary sinus full haziness with mass effect were associated with the presence of MFB. When performing a surgery for patients with MFB, fungus ball has a characteristic gritty, matted gross appearance to the surgeon, which corresponds to the fuzzy appearance on the CT scan. From the chronic disease process, bone erosion is possible as one study had shown that disease duration was longer in patients presenting with bone erosion compared to those without bone erosion [19]. Therefore, even in the absence of intralesional hyperdensity, one should clinically suspect MFB when such radiologic features are present.
Among demographic data, age was the only clue to a fungus ball. Endodontic treatment is known as one of the possible risk factors for development of a paranasal fungus ball. Zinc in the root canal fillings may stimulate fungal growth within the maxillary sinus [10]. Despite the lack of evidence, history of endodontic treatment among the older or decreased mucociliary function in the elderly are thought to be associated with fungus ball [3]. Interestingly, our decision tree demonstrated that young patients under age 33 years having fuzzy appearance without any intralesional hyperdensity favored MFB. One possible explanation is the genetic susceptibility in these patients. A recent study discovered an association of single nucleotide polymorphism (rs7203904 [C>G]) to sinonasal fungal ball. This SNP has been reported to be associated with epithelial E-cadherin expression [20]. Therefore pathogenesis of MFB in young age needs to be further evaluated.
In our study, there was no significant difference in multivariate analysis and regression tree model for female predominance. Therefore, radiological characteristics and age may have affected more than the gender difference. However, as was noticed in the previous studies [1,10,14], and from the univariate analysis, there seems to be a female predominance. The reason for gender predominance is still unclear.
It is known that fungus ball is also associated with the presence of marginally immunocompromised patients [2,14,21-23]. Despite the fact that comorbid DM was not significantly associated with the fungus ball in our study, other studies have reported DM as a frequent comorbid disease in patients with fungus ball [2]. Therefore, more studies should be analyzed.
Old age and other characteristic non-intralesional hyperdensity lesions had been already demonstrated to be associated with MFB in the previous studies [2,3,7]. However, as far as we know, no study had represented that these clinical features may increase the preoperative diagnostic accuracy. This study demonstrated a decision tree to determine the optimal algorithm in the diagnosis of sinonasal fungus ball from the preoperative clinical parameters. Although through multivariate logistic regression, significant clinical features associated with MFB were identified, it is difficult to develop a diagnostic algorithm from these features since we cannot estimate the optimal hierarchy of these clinical variables solely based on the calculated OR. However, through the regression tree analysis, it became clearer. As independent variables were added through models 1 to 3, tentative age criteria became precise, and the overall diagnostic accuracy became higher.
Presence of mucosal thickening was more commonly associated with CMS; however, this was not selected as a feature in the regression tree analysis. This is a mixture of heterogenous radiological characteristics, such as mucosal thickening, air-fluid level, and therefore more detailed categorization of such radiological features may increase the overall accuracy.
There are several limitations in this study. Previous study reported unilateral CRS being the most common etiology in unilateral sinus disease [24]; however, in our series, MFB was more common than CMS. In our study, we have excluded patients having a periapical lucency or bony dehiscence as these patients were regarded to have CMS due to odontogenic causes. However, not all CMS with periapical lucency or bony dehiscence are caused by odontogenic factors. Also, patients with remaining disease despite medical therapy tend to have ESS which may explain the higher incidence of MFB (which is resistant to medical therapy). Therefore, this study entails a selection bias that subjects who are less likely to enter the study may have been under-represented. However, at the same time, inclusion of all these patients may increase an overall type I error. In addition, some studies demonstrated a correlation between odontogenic factors and MFB [25,26]. However, these factors had not been evaluated in current study. This is a single center study with a relatively small number of patients that geographical and ethnic factors may have affected. Therefore, further studies are required on a larger scale and additional radiological parameters in the future.
Intralesional hyperdensity in the maxillary sinus suggested a fungus ball in patients with unilateral maxillary sinusitis. However, in the absence of intralesional hyperdensity, old age and other radiological features such as presence of lobulated protruding lesion may also suggest a fungus ball. Physicians should be aware of such findings in the management of patients presenting with unilateral maxillary sinusitis.
▪ The intralesional hyperdensity lesion on the computed tomography (CT) scan is highly specific for maxillary sinus fungal ball; however, a significant proportion of patients may not present with intralesional hyperdensity.
▪ Age and characteristic non-intralesional hyperdensity lesions, the presence of an irregular protruding lesion and maxillary sinus full haziness with mass effect in the CT scans are associated with the presence of maxillary sinus fungal ball.
▪ Demographic parameter and characteristic non-calcified lesion increase the overall diagnostic accuracy of maxillary sinus fungal ball.
ACKNOWLEDGMENTS
This work has been awarded a travel grant funded by the Korean American Otolaryngology Society (KAOS).
REFERENCES
1. Dufour X, Kauffmann-Lacroix C, Ferrie JC, Goujon JM, Rodier MH, Klossek JM. Paranasal sinus fungus ball: epidemiology, clinical features and diagnosis: a retrospective analysis of 173 cases from a single medical center in France, 1989-2002. Med Mycol. 2006; Feb. 44(1):61–7.

2. Jiang RS, Huang WC, Liang KL. Characteristics of sinus fungus ball: a unique form of rhinosinusitis. Clin Med Insights Ear Nose Throat. 2018; Aug. 11:1179550618792254.

3. Nomura K, Asaka D, Nakayama T, Okushi T, Matsuwaki Y, Yoshimura T, et al. Sinus fungus ball in the Japanese population: clinical and imaging characteristics of 104 cases. Int J Otolaryngol. 2013; 2013:731640.

4. Yoon YH, Xu J, Park SK, Heo JH, Kim YM, Rha KS. A retrospective analysis of 538 sinonasal fungus ball cases treated at a single tertiary medical center in Korea (1996-2015). Int Forum Allergy Rhinol. 2017; Nov. 7(11):1070–5.

5. deShazo RD, O’Brien M, Chapin K, Soto-Aguilar M, Swain R, Lyons M, et al. Criteria for the diagnosis of sinus mycetoma. J Allergy Clin Immunol. 1997; Apr. 99(4):475–85.

6. Chen JC, Ho CY. The significance of computed tomographic findings in the diagnosis of fungus ball in the paranasal sinuses. Am J Rhinol Allergy. 2012; Mar-Apr. 26(2):117–9.

7. Ho CF, Lee TJ, Wu PW, Huang CC, Chang PH, Huang YL, et al. Diagnosis of a maxillary sinus fungus ball without intralesional hyperdensity on computed tomography. Laryngoscope. 2019; May. 129(5):1041–5.

8. Dhong HJ, Jung JY, Park JH. Diagnostic accuracy in sinus fungus balls: CT scan and operative findings. Am J Rhinol. 2000; Jul-Aug. 14(4):227–31.

9. Seo YJ, Kim J, Kim K, Lee JG, Kim CH, Yoon JH. Radiologic characteristics of sinonasal fungus ball: an analysis of 119 cases. Acta Radiol. 2011; Sep. 52(7):790–5.

10. Klossek JM, Serrano E, Peloquin L, Percodani J, Fontanel JP, Pessey JJ. Functional endoscopic sinus surgery and 109 mycetomas of paranasal sinuses. Laryngoscope. 1997; Jan. 107(1):112–7.

11. McCarty JL, David RM, Lensing SY, Samant RS, Kumar M, Van Hemert RL, et al. Root cause analysis: an examination of odontogenic origins of acute maxillary sinusitis in both immunocompetent & immunocompromised patients. J Comput Assist Tomogr. 2017; May/Jun. 41(3):484–8.
12. Smith TC, Frank E. Introducing machine learning concepts with WEKA. Methods Mol Biol. 2016; 1418:353–78.

13. Kim JS, So SS, Kwon SH. The increasing incidence of paranasal sinus fungus ball: a retrospective cohort study in two hundred forty-five patients for fifteen years. Clin Otolaryngol. 2017; Feb. 42(1):175–9.

14. Ferguson BJ. Fungus balls of the paranasal sinuses. Otolaryngol Clin North Am. 2000; Apr. 33(2):389–98.

15. Jiang Z, Zhang K, Huang W, Yuan Q. A preliminary study on sinus fungus ball with MicroCT and X-ray fluorescence technique. PLoS One. 2016; Mar. 11(3):e0148515.

16. Willinger B, Obradovic A, Selitsch B, Beck-Mannagetta J, Buzina W, Braun H, et al. Detection and identification of fungi from fungus balls of the maxillary sinus by molecular techniques. J Clin Microbiol. 2003; Feb. 41(2):581–5.

17. Wilson D, Citiulo F, Hube B. Zinc exploitation by pathogenic fungi. PLoS Pathog. 2012; Dec. 8(12):e1003034.

18. Eide DJ. Homeostatic and adaptive responses to zinc deficiency in Saccharomyces cerevisiae. J Biol Chem. 2009; Jul. 284(28):18565–9.

19. Zhu H, Zhang W, Guan J, Ye H, Su K. CT imaging and clinical features of sinus fungus ball with bone erosion. J Nat Sci. 2015; 1(4):e69.
20. Ierodiakonou D, Postma DS, Koppelman GH, Boezen HM, Gerritsen J, Ten Hacken N, et al. E-cadherin gene polymorphisms in asthma patients using inhaled corticosteroids. Eur Respir J. 2011; Nov. 38(5):1044–52.

21. Robey AB, O’Brien EK, Richardson BE, Baker JJ, Poage DP, Leopold DA. The changing face of paranasal sinus fungus balls. Ann Otol Rhinol Laryngol. 2009; Jul. 118(7):500–5.

24. Paz Silva M, Pinto JM, Corey JP, Mhoon EE, Baroody FM, Naclerio RM. Diagnostic algorithm for unilateral sinus disease: a 15-year retrospective review. Int Forum Allergy Rhinol. 2015; Jul. 5(7):590–6.
Fig. 1.
Representative radiological findings. (A) Irregular lobulated protruding lesion (fuzzy appearance). (B) Maxillary sinus full haziness without mass effect. (C) Maxillary sinus full haziness with mass effect. (D) Others (mucosal thickening in this case).
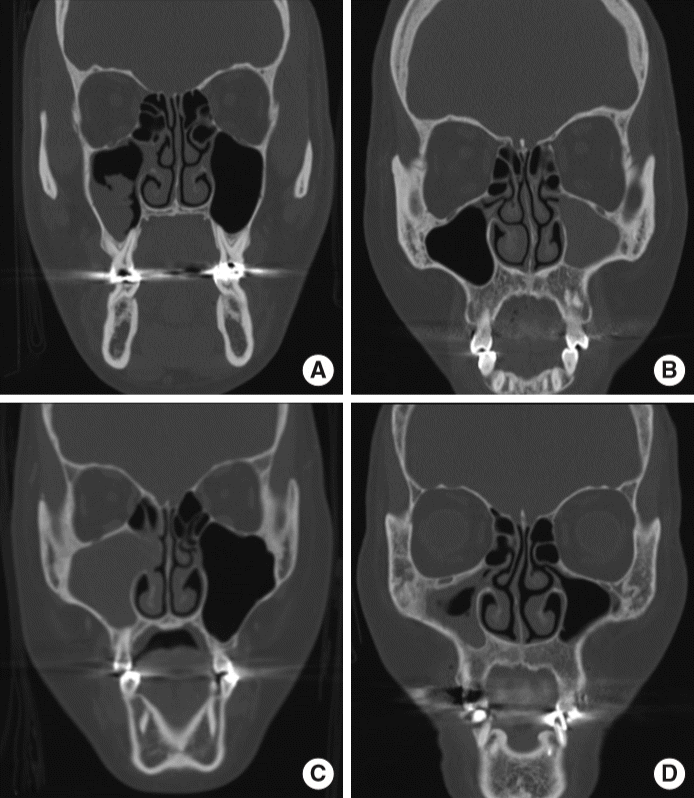
Fig. 2.
Enrollment flowchart. ESS, endoscopic sinus surgery; CRS, chronic rhinosinusitis; CMS, chronic maxillary sinusitis; MFB, maxillary sinus fungus ball. a)Postoperative maxillary cyst, inverted papilloma, malignant tumor, or antrochoanal polyps, etc.
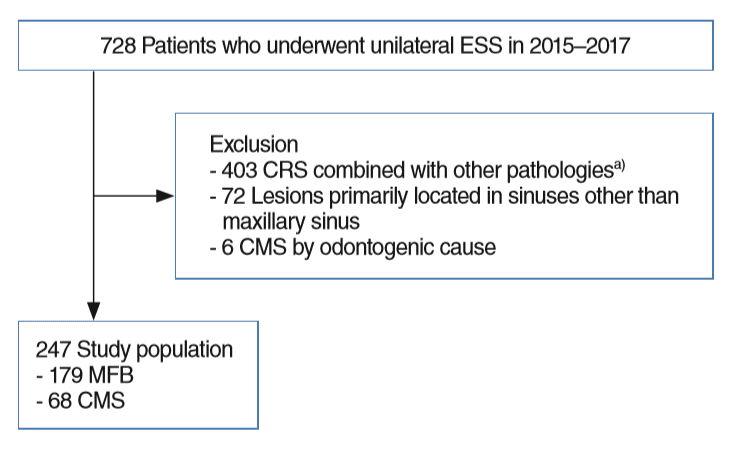
Fig. 3.
Regression tree model for diagnosis. (A) Model 1. (B) Model 2. (C) Model 3. Model 1 only involved intralesional hyperdensity as a variable; model 2 added demographic data; and model 3 included intralesional hyperdensity and demographic data, as well as radiological features (lobulated protruding lesion and full haziness with mass effect). Model 1: area under the curve, 0.853; accuracy, 81.78%; model 2: 0.880, 79.76%; model 3: 0.904, 88.66%, respectively. MFB, maxillary sinus fungus ball; CMS, chronic maxillary sinusitis.
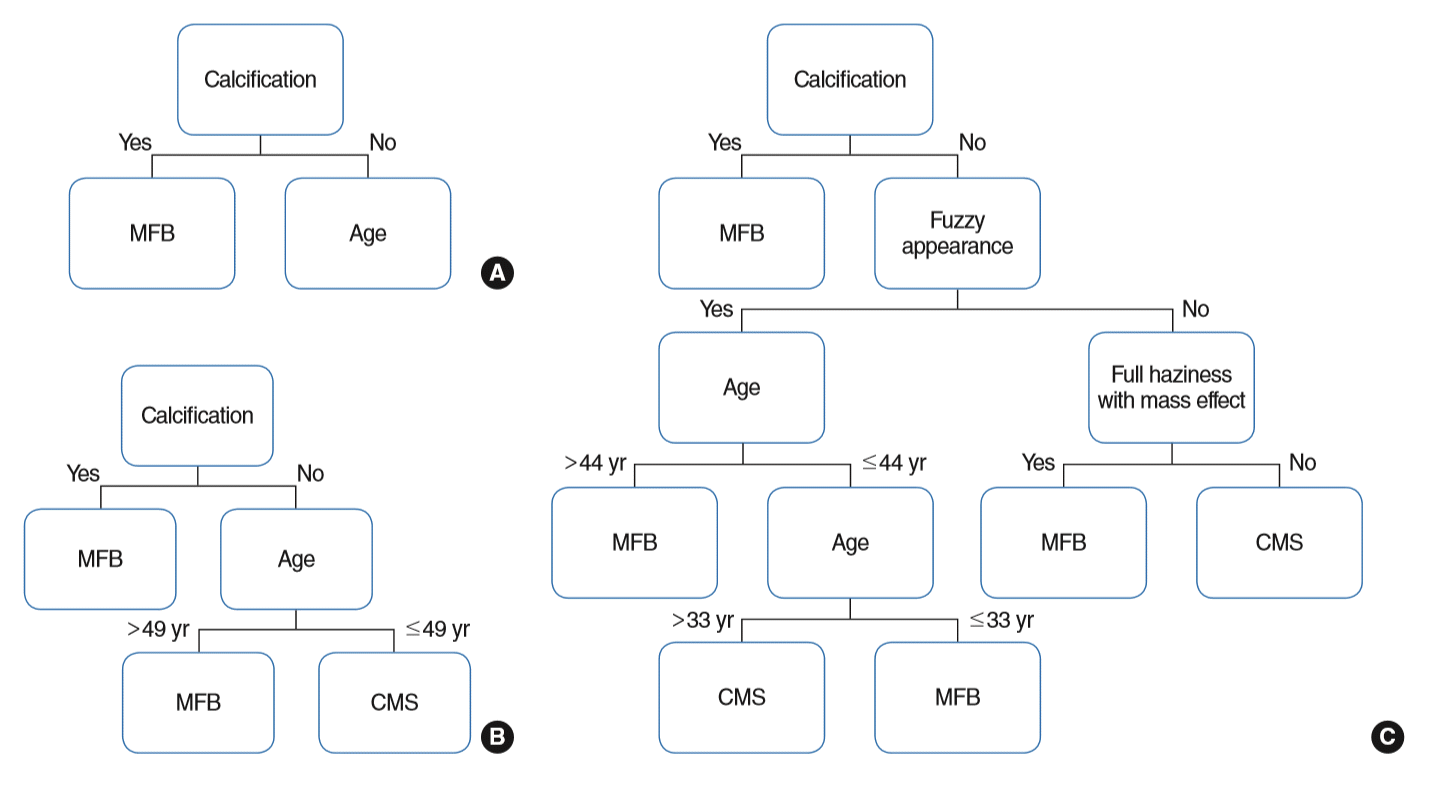
Table 1.
Demographic characteristics
| Variable | Total (n=247) | MFB (n=179) | CMS (n=68) | P-value |
|---|---|---|---|---|
| Age (yr) | 57.5±15.8 | 61.1±13.4 | 48.2±17.9 | <0.001 |
| Male | 94 (38.1) | 57 (31.8) | 37 (54.4) | 0.002 |
| Diabetes mellitus | 12 (4.9) | 10 (5.6) | 2 (2.9) | 0.520 |
Table 2.
Radiologic findings
| Variable | MFB (n=179) | CMS (n=68) | P-value |
|---|---|---|---|
| Intralesional hyperdensity | 134 (74.9) | 0 | <0.001 |
| Fuzzy appearance | 55 (69.1) | 5 (7.4) | <0.001 |
| Full haziness without mass effecta) | 20 (11.2) | 10 (14.7) | 0.513 |
| Full haziness with mass effect | 60 (33.5) | 6 (8.8) | <0.001 |
| Othersb) | 45 (25.1) | 46 (67.6) | <0.001 |
Table 3.
Multivariate analysis of clinical characteristics associated with maxillary fungal ball




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download