1. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016; Jan. 26(1):1–133.

2. Chandrasekhar SS, Randolph GW, Seidman MD, Rosenfeld RM, Angelos P, Barkmeier-Kraemer J, et al. Clinical practice guideline: improving voice outcomes after thyroid surgery. Otolaryngol Head Neck Surg. 2013; Jun. 148(6 Suppl):S1–37.
3. Munch S, deKryger L. A piece of my mind. Moral wounds: complicated complications. JAMA. 2001; Mar. 285(9):1131–2.
4. Jeannon JP, Orabi AA, Bruch GA, Abdalsalam HA, Simo R. Diagnosis of recurrent laryngeal nerve palsy after thyroidectomy: a systematic review. Int J Clin Pract. 2009; Apr. 63(4):624–9.

5. Riddell V. Thyroidectomy: prevention of bilateral recurrent nerve palsy. Results of identification of the nerve over 23 consecutive years (1946-69) with a description of an additional safety measure. Br J Surg. 1970; Jan. 57(1):1–11.

6. Hermann M, Alk G, Roka R, Glaser K, Freissmuth M. Laryngeal recurrent nerve injury in surgery for benign thyroid diseases: effect of nerve dissection and impact of individual surgeon in more than 27,000 nerves at risk. Ann Surg. 2002; Feb. 235(2):261–8.
7. Chiang FY, Wang LF, Huang YF, Lee KW, Kuo WR. Recurrent laryngeal nerve palsy after thyroidectomy with routine identification of the recurrent laryngeal nerve. Surgery. 2005; Mar. 137(3):342–7.

8. Randolph GW, Dralle H; International Intraoperative Monitoring Study Group, Abdullah H, Barczynski M, Bellantone R, et al. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope. 2011; Jan. 121 Suppl 1:S1–16.

9. Terris DJ, Snyder S, Carneiro-Pla D, Inabnet WB 3rd, Kandil E, Orloff L, et al. American Thyroid Association statement on outpatient thyroidectomy. Thyroid. 2013; Oct. 23(10):1193–202.

10. Chen AY, Bernet VJ, Carty SE, Davies TF, Ganly I, Inabnet WB 3rd, et al. American Thyroid Association statement on optimal surgical management of goiter. Thyroid. 2014; Feb. 24(2):181–9.

11. Chiang FY, Lee KW, Chen HC, Chen HY, Lu IC, Kuo WR, et al. Standardization of intraoperative neuromonitoring of recurrent laryngeal nerve in thyroid operation. World J Surg. 2010; Feb. 34(2):223–9.

12. Sung ES, Lee BJ. Development of the novel intraoperative neuromonitoring for thyroid surgery. Int J Thyroid. 2018; 11(2):109–16.

13. Sung ES, Lee JC, Shin SC, Kwon HG, Kim MS, Kim DJ, et al. Development of a novel intraoperative neuromonitoring system using a surface pressure sensor to detect muscle movement: a rabbit model study. Clin Exp Otorhinolaryngol. 2019; May. 12(2):217–23.

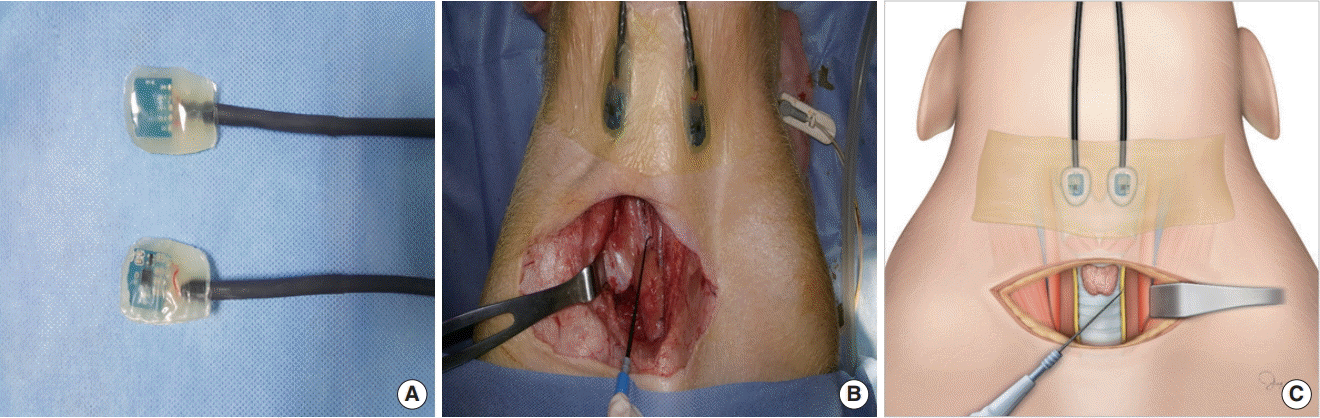
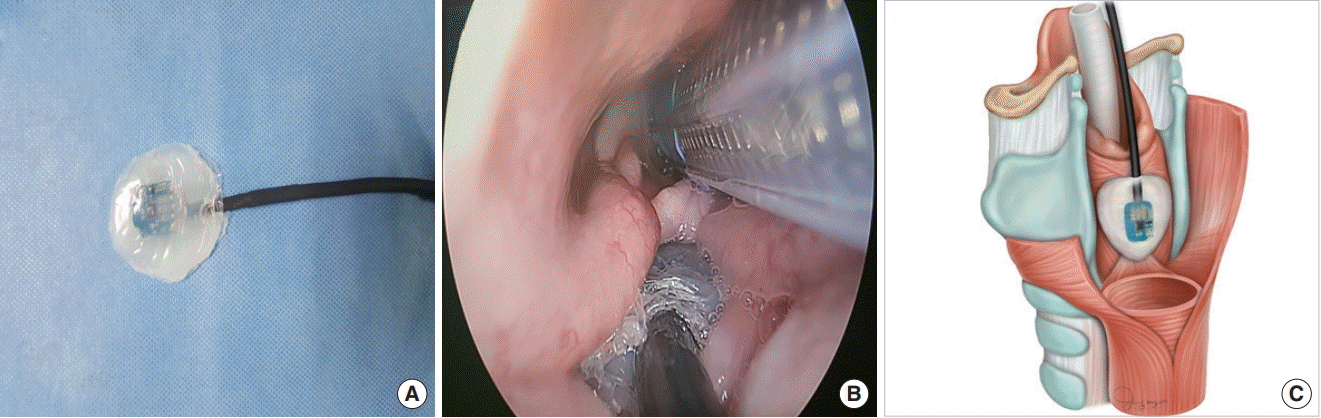
14. Sung ES, Lee JC, Kim SH, Shin SC, Jung DW, Lee BJ. Development of an attachable endoscopic nerve stimulator for intraoperative neuromonitoring during endoscopic or robotic thyroidectomy. Otolaryngol Head Neck Surg. 2018; Mar. 158(3):465–8.

15. Shin SC, Sung ES, Choi SW, Kim SD, Jung DW, Kim SH, et al. Feasibility and safety of nerve stimulator attachment to energy-based devices: a porcine model study. Int J Surg. 2017; Dec. 48:155–9.

16. Khan A, Pearlman RC, Bianchi DA, Hauck KW. Experience with two types of electromyography monitoring electrodes during thyroid surgery. Am J Otolaryngol. 1997; Mar-Apr. 18(2):99–102.

17. Tschopp KP, Gottardo C. Comparison of various methods of electromyographic monitoring of the recurrent laryngeal nerve in thyroid surgery. Ann Otol Rhinol Laryngol. 2002; Sep. 111(9):811–6.

18. Rea JL, Khan A. Clinical evoked electromyography for recurrent laryngeal nerve preservation: use of an endotracheal tube electrode and a postcricoid surface electrode. Laryngoscope. 1998; Sep. 108(9):1418–20.

19. Marcus B, Edwards B, Yoo S, Byrne A, Gupta A, Kandrevas J, et al. Recurrent laryngeal nerve monitoring in thyroid and parathyroid surgery: the University of Michigan experience. Laryngoscope. 2003; Feb. 113(2):356–61.

20. Liddy W, Barber SR, Lin BM, Kamani D, Kyriazidis N, Lawson B, et al. Monitoring of the posterior cricoarytenoid muscle represents another option for neural monitoring during thyroid surgery: normative vagal and recurrent laryngeal nerve posterior cricoarytenoid muscle electromyographic data. Laryngoscope. 2018; Jan. 128(1):283–9.

21. Wu CW, Chiang FY, Randolph GW, Dionigi G, Kim HY, Lin YC, et al. Feasibility of intraoperative neuromonitoring during thyroid surgery using transcartilage surface recording electrodes. Thyroid. 2018; Nov. 28(11):1508–16.

22. Wu CW, Chiang FY, Randolph GW, Dionigi G, Kim HY, Lin YC, et al. Transcutaneous recording during intraoperative neuromonitoring in thyroid surgery. Thyroid. 2018; Nov. 28(11):1500–7.

23. Dralle H, Sekulla C, Lorenz K, Brauckhoff M, Machens A; German IONM Study Group. Intraoperative monitoring of the recurrent laryngeal nerve in thyroid surgery. World J Surg. 2008; Jul. 32(7):1358–66.

24. Bai B, Chen W. Protective effects of intraoperative nerve monitoring (IONM) for recurrent laryngeal nerve injury in thyroidectomy: meta-analysis. Sci Rep. 2018; May. 8(1):7761.

25. Sanabria A, Ramirez A, Kowalski LP, Silver CE, Shaha AR, Owen RP, et al. Neuromonitoring in thyroidectomy: a meta-analysis of effectiveness from randomized controlled trials. Eur Arch Otorhinolaryngol. 2013; Aug. 270(8):2175–89.

26. Lifante JC, McGill J, Murry T, Aviv JE, Inabnet WB 3rd. A prospective, randomized trial of nerve monitoring of the external branch of the superior laryngeal nerve during thyroidectomy under local/regional anesthesia and IV sedation. Surgery. 2009; Dec. 146(6):1167–73.

27. Barczynski M, Konturek A, Cichon S. Randomized clinical trial of visualization versus neuromonitoring of recurrent laryngeal nerves during thyroidectomy. Br J Surg. 2009; Mar. 96(3):240–6.
28. Higgins TS, Gupta R, Ketcham AS, Sataloff RT, Wadsworth JT, Sinacori JT. Recurrent laryngeal nerve monitoring versus identification alone on post-thyroidectomy true vocal fold palsy: a meta-analysis. Laryngoscope. 2011; May. 121(5):1009–17.

29. Dionigi G, Boni L, Rovera F, Bacuzzi A, Dionigi R. Neuromonitoring and video-assisted thyroidectomy: a prospective, randomized casecontrol evaluation. Surg Endosc. 2009; May. 23(5):996–1003.

30. Chan WF, Lo CY. Pitfalls of intraoperative neuromonitoring for predicting postoperative recurrent laryngeal nerve function during thyroidectomy. World J Surg. 2006; May. 30(5):806–12.

31. Chiang FY, Lu IC, Kuo WR, Lee KW, Chang NC, Wu CW. The mechanism of recurrent laryngeal nerve injury during thyroid surgery: the application of intraoperative neuromonitoring. Surgery. 2008; Jun. 143(6):743–9.
32. Dionigi G, Bacuzzi A, Boni L, Rovera F, Dionigi R. What is the learning curve for intraoperative neuromonitoring in thyroid surgery. Int J Surg. 2008; 6 Suppl 1:S7–12.

33. Kim HY, Tufano RP, Randolph G, Barczynski M, Wu CW, Chiang FY, et al. Impact of positional changes in neural monitoring endotracheal tube on amplitude and latency of electromyographic response in monitored thyroid surgery: results from the porcine experiment. Head Neck. 2016; Apr. 38 Suppl 1:E1004–8.

34. Wu CW, Wang MH, Chen CC, Chen HC, Chen HY, Yu JY, et al. Loss of signal in recurrent nerve neuromonitoring: causes and management. Gland Surg. 2015; Feb. 4(1):19–26.
35. Chiang FY, Lu IC, Chang PY, Dionigi G, Randolph GW, Sun H, et al. Comparison of EMG signals recorded by surface electrodes on endotracheal tube and thyroid cartilage during monitored thyroidectomy. Kaohsiung J Med Sci. 2017; Oct. 33(10):503–9.

36. Randolph GW, Kobler JB, Wilkins J. Recurrent laryngeal nerve identification and assessment during thyroid surgery: laryngeal palpation. World J Surg. 2004; Aug. 28(8):755–60.

37. Puram SV, Chow H, Wu CW, Heaton JT, Kamani D, Gorti G, et al. Posterior cricoarytenoid muscle electrophysiologic changes are predictive of vocal cord paralysis with recurrent laryngeal nerve compressive injury in a canine model. Laryngoscope. 2016; Dec. 126(12):2744–51.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download