INTRODUCTION
MATERIALS AND METHODS
Experimental animals
Preparation of H. fusiformis extract
Experimental protocols
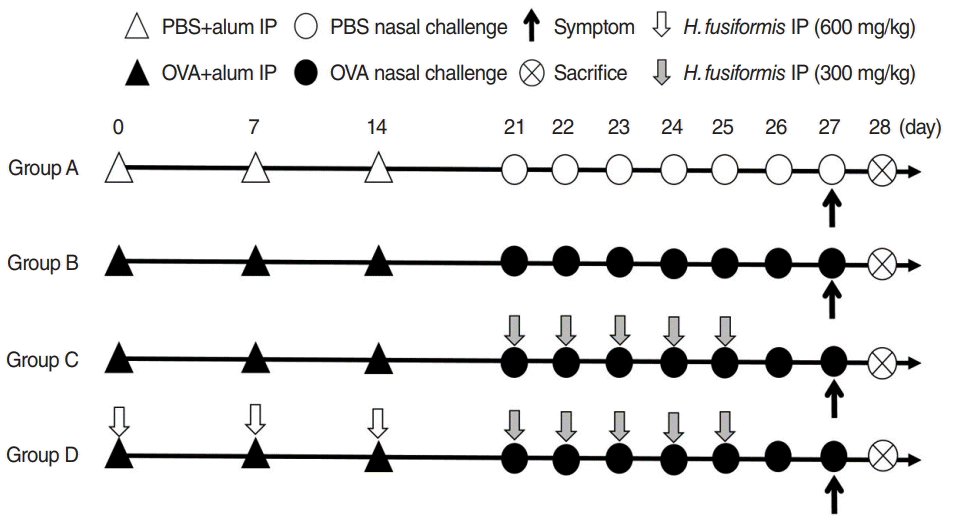 | Fig. 1.Schematic representation of the experimental protocol. (General sensitization) BALB/c mice were sensitized with 25 μg of ovalbumin (OVA) and 2 mg of aluminum hydroxide gel on days 0, 7, and 14. (Local sensitization) all mice except for group A received intranasal challenge with OVA from day 21 to 27. In group C, mice received Hizikia fusiformis (H. fusiformis, 300 mg/kg) 3 hours before intranasal OVA challenge from day 21 to 25. In group D, mice received H. fusiformis (600 mg/kg) 3 hours before intraperitoneal OVA administration on days 0, 7, and 14 and H. fusiformis (300 mg/kg) 3 hours before intranasal OVA challenge. PBS, phosphate-buffered saline; IP, intraperitoneal injection. |
Nasal symptom scores
Histologic assessment
Measurement of serum levels of OVA-specific IgE, IgG1, and IgG2a
Real-time polymerase chain reaction for IL-5, IL-10, IFN-γ, and FOXP3 in the nasal mucosa
Table 1.
Measurement of cytokines (IL-4, IL-5, IL-13, TNF-α, and IL-10) levels in splenocyte culture
Flow cytometry analysis
Statistical analysis
RESULTS
Reduced allergic symptoms and serum OVA-specific IgE, IgG1, and IgG2a Abs after H. fusiformis treatment
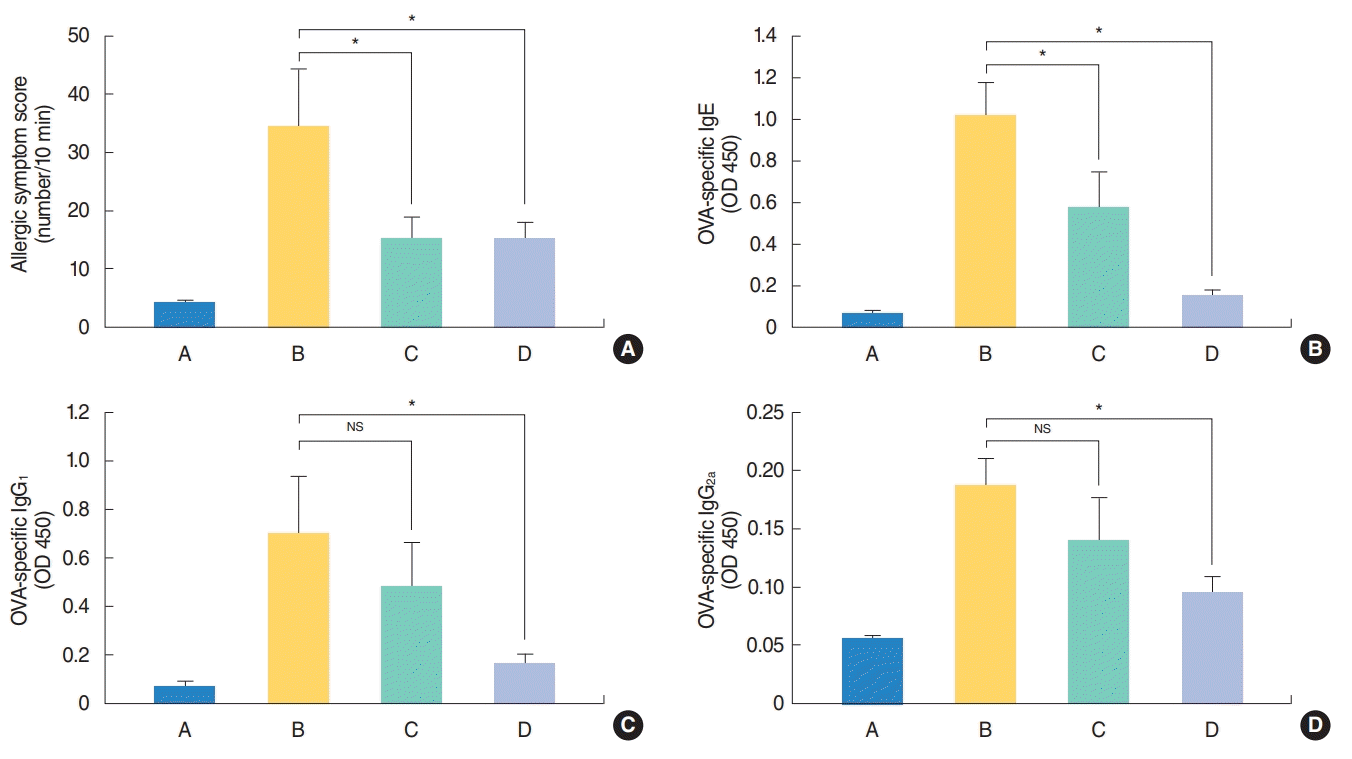 | Fig. 2.Effect of Hizikia fusiformis on levels of (A) allergic symptom score and (B-D) serum ovalbumin (OVA)-specific immunoglobulins in each group. Administration of H. fusiformis suppressed allergic symptoms including rubbing and sneezing, and serum immunoglobulin E (IgE) (B), IgG1 (C), and IgG2a (D) levels. A, control group; B, allergic rhinitis group; C, H. fusiformis treatment group during nasal challenging period; D, H. fusiformis treatment group during general sensitization and nasal challenging period; NS, not significant. *P<0.05. |
Systemic effects of H. fusiformis evident from cytokine production and flow cytometric analysis of CD4+CD25+FOXP3+ T cells from splenocytes
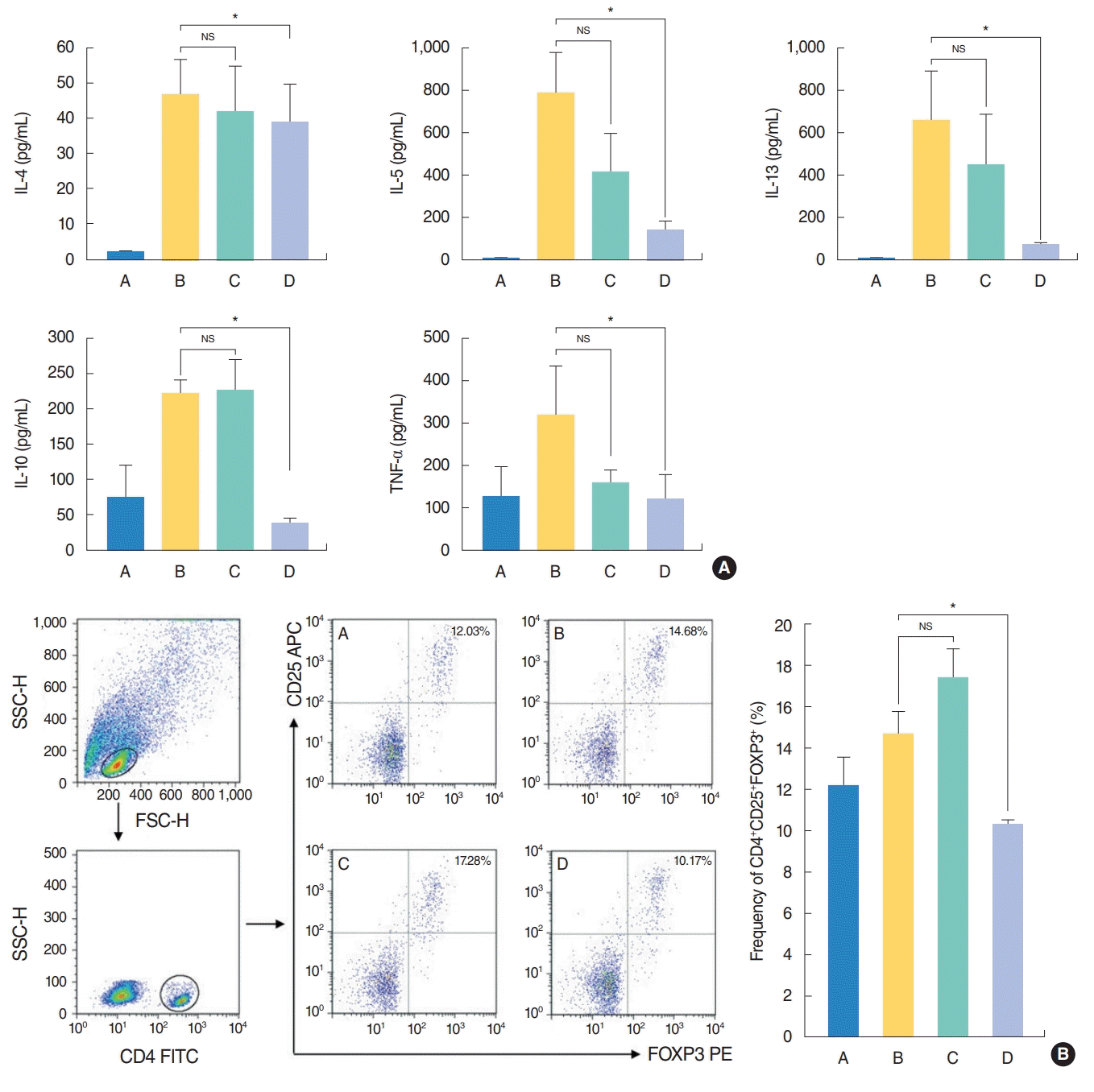 | Fig. 3.Effect of Hizikia fusiformis on the (A) production of Th1/Th2 cytokines and (B) induction of regulatory T cell subgroups in spleen cell culture. (A) In spleen cell culture supernatant, interleukin 4 (IL-4), IL-5, IL-13, IL-10 and tumor necrosis factor alpha (TNF-α) concentrations were decreased significantly in group D compared with the group B. (B) The frequency of CD4+CD25+FOXP3+ was decreased significantly in group D when the splenocyte cultured to OVA compared to the positive control group B. However, others did not show significant change after treatment. Experiments were performed three times. A, control group; B, allergic rhinitis group; C, H. fusiformis treatment group during nasal challenging period; D, H. fusiformis treatment group during general sensitization and nasal challenging period; NS, not significant; SSC-H, side scatter height; FSC-H, forward scatter height; FITC, fluorescein isothiocyanate; APC, allophycocyanine; PE, phycoerythrin. *P<0.05. |
Local effects of H. fusiformis evident from cytokine mRNA expression
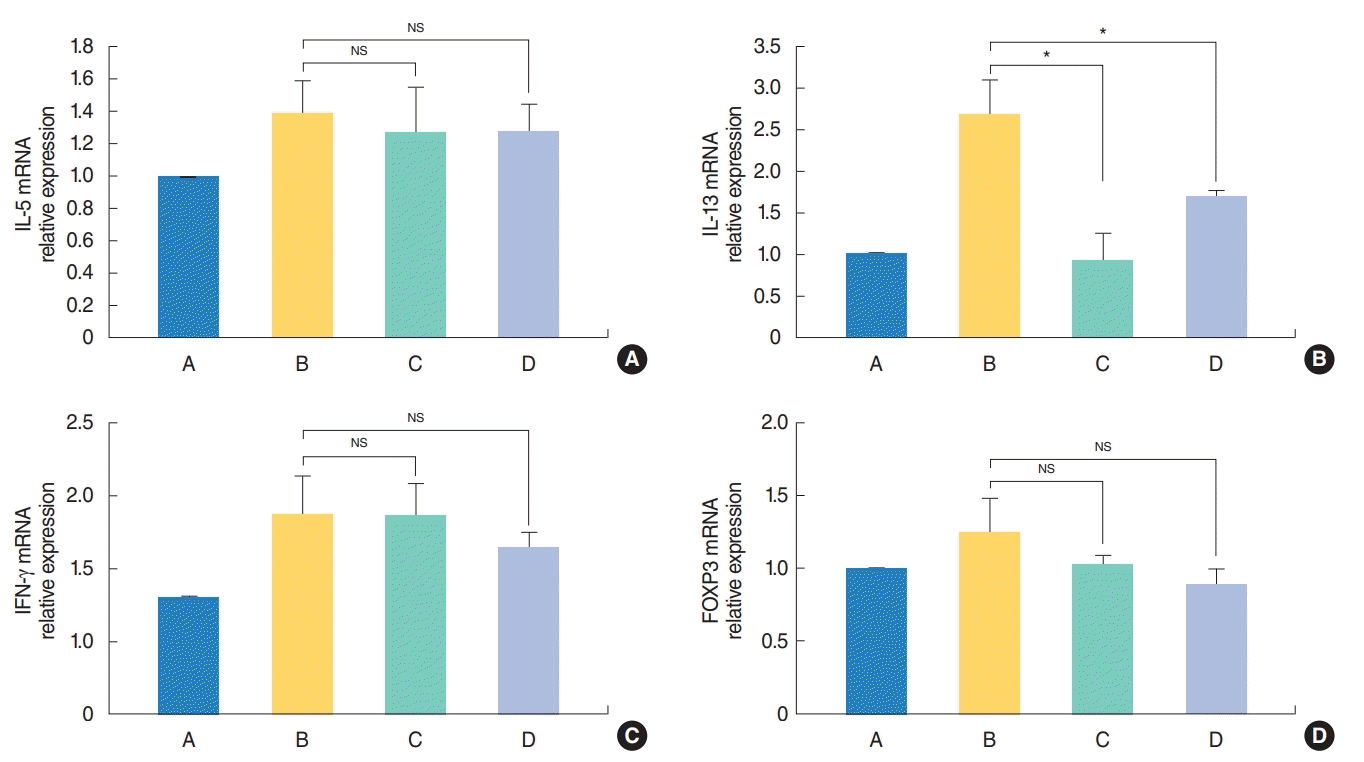 | Fig. 4.Effect of Hizikia fusiformis on the nasal tissue cytokine expression. Local cytokine mRNA expression levels in the nasal mucosa were evaluated using real-time polymerase chain reaction after H. fusiformis administration. Transcriptional activity of interleukin 13 (IL-13) decreased significantly (B); however, those of IL-5 (A), interferon γ (IFN-γ; C), and FOXP3 (D) did not significantly change in the groups C and D compared with the positive control group B. A, control group; B, allergic rhinitis group; C, H. fusiformis treatment group during nasal challenging period; D, H. fusiformis treatment group during general sensitization and nasal challenging period; NS, not significant. *P<0.05 |
Reduced eosinophil/goblet cell counts in the nasal mucosa after H. fusiformis treatment
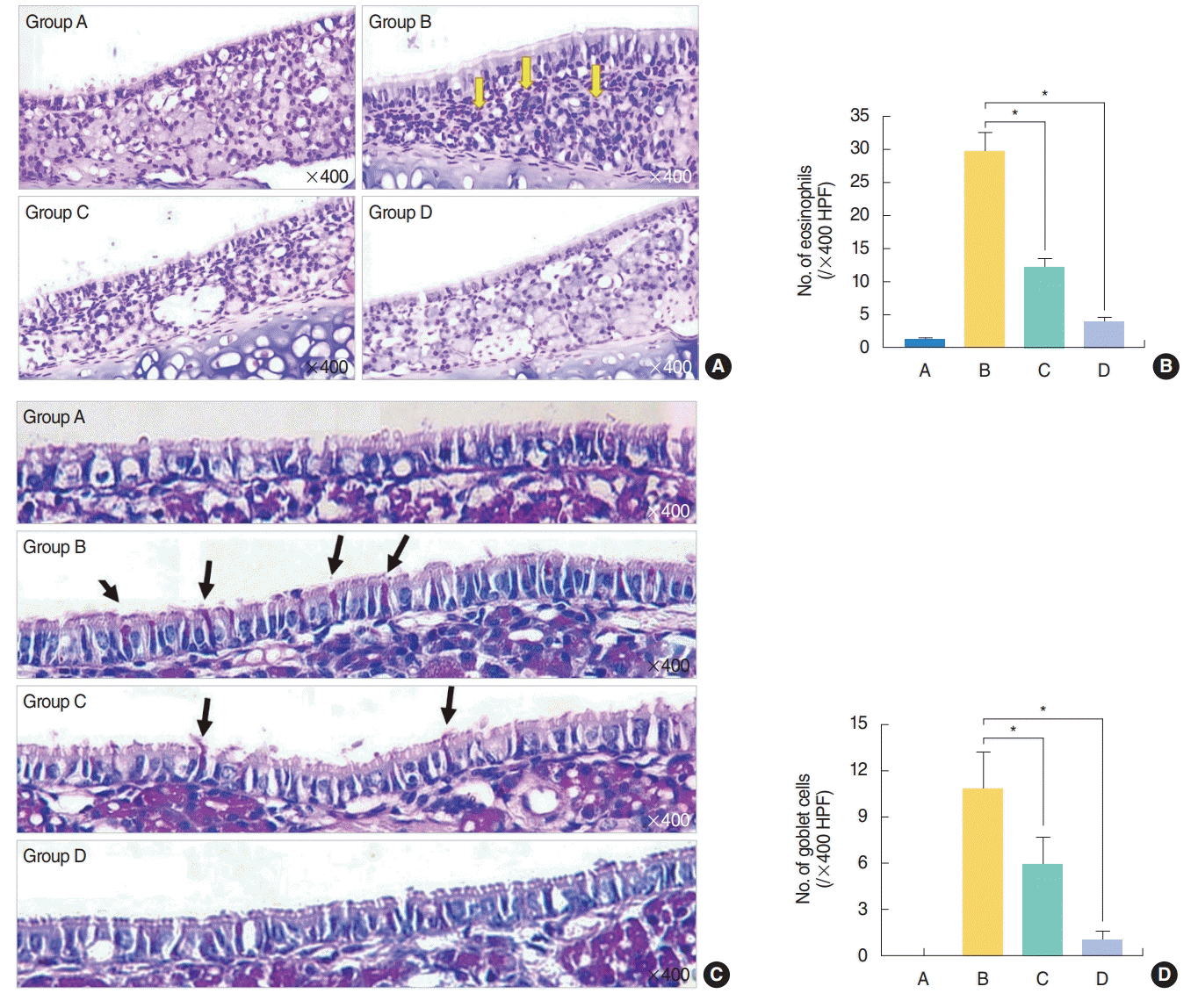 | Fig. 5.Histopathological findings. (A, B) Eosinophil infiltration in the nasal mucosa. (C, D) Goblet cell hyperplasia in the nasal epithelium. (A) Subepithelial inflammatory cell infiltration including eosinophil was evident in group B and decreased in Hizikia fusiformis treatment groups C and D (Sirius red stain, ×400; yellow arrows, eosinophils). (B) Eosinophil count was significantly reduced in treatment groups C and D compared with the positive group B. (C) Increased number of goblet cell in epithelial layer was identified in group B (black arrows, goblet cells). (D) Systemic administration of H. fusiformis suppress goblet cell hyperplasia in groups C and D compared with the positive control group B (Periodic acid-Schiff stain, ×400). A, control group; B, allergic rhinitis group; C, H. fusiformis treatment group during nasal challenging period; D, H. fusiformis treatment group during general sensitization and nasal challenging period; HPF, high power field. *P<0.05. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download