1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010; Dec. 127(12):2893–917.

2. Chu EA, Kim YJ. Laryngeal cancer: diagnosis and preoperative work- up. Otolaryngol Clin North Am. 2008; Aug. 41(4):673–95.
3. Laoufi S, Mirghani H, Janot F, Hartl DM. Voice quality after treatment of T1a glottic cancer. Laryngoscope. 2014; Jun. 124(6):1398–401.

4. Lee HH, Lee KD, Lee HK, Pyo KB, Ahn KM, Lee YS. Treatment results and voice analysis after laser cordectomy for glottic T1 cancer. Korean J Otorhinolaryngol Head Neck Surg. 2002; Aug. 45(8):805–10.
5. Brandenburg JH. Laser cordotomy versus radiotherapy: an objective cost analysis. Ann Otol Rhinol Laryngol. 2001; Apr. 110(4):312–8.

6. Higgins KM, Shah MD, Ogaick MJ, Enepekides D. Treatment of early-stage glottic cancer: meta-analysis comparison of laser excision versus radiotherapy. J Otolaryngol Head Neck Surg. 2009; Dec. 38(6):603–12.
7. Greulich MT, Parker NP, Lee P, Merati AL, Misono S. Voice outcomes following radiation versus laser microsurgery for T1 glottic carcinoma: systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2015; May. 152(5):811–9.
8. Huang G, Luo M, Zhang J, Liu H. The voice quality after laser surgery versus radiotherapy of T1a glottic carcinoma: systematic review and meta-analysis. Onco Targets Ther. 2017; May. 10:2403–10.

9. Hong YT, Park MJ, Hong KH. Characteristics of speech production in patients with T1 glottic cancer who underwent laser cordectomy or radiotherapy. Logoped Phoniatr Vocol. 2018; Oct. 43(3):120–8.

10. Luo CM, Fang TJ, Lin CY, Chang JT, Liao CT, Chen IH, et al. Transoral laser microsurgery elevates fundamental frequency in early glottic cancer. J Voice. 2012; Sep. 26(5):596–601.

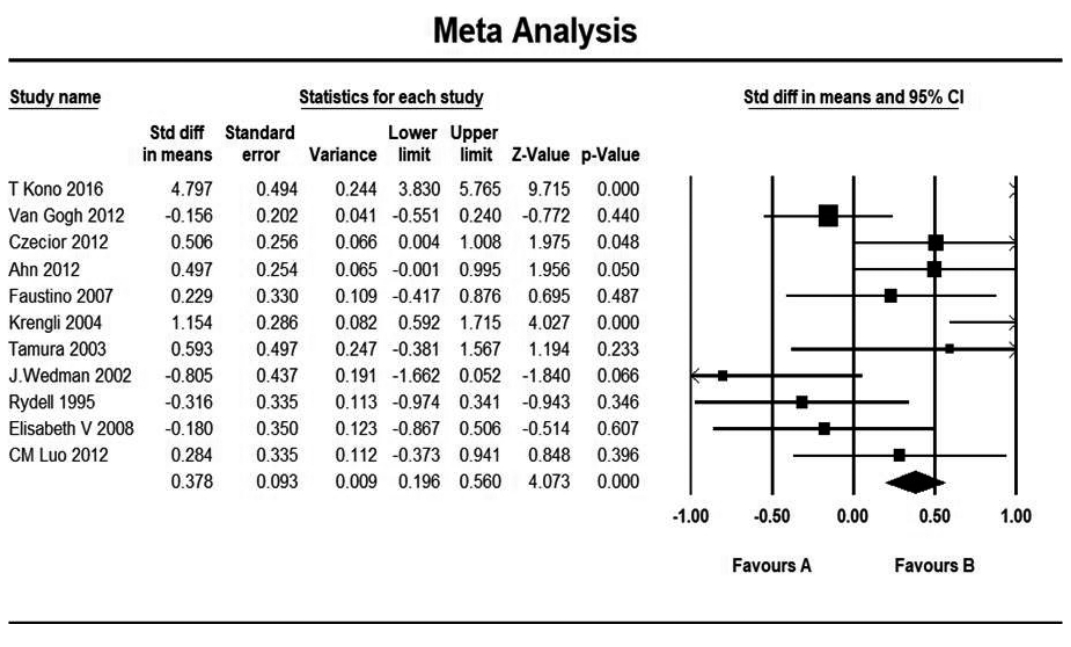
11. Czecior E, Orecka B, Pawlas P, Mrowka-Kata K, Namyslowski G, Skladowski K, et al. Comparative assessment of the voice in patients treated for early glottis cancer by laser cordectomy or radiotherapy. Otolaryngol Pol. 2012; Nov-Dec. 66(6):407–12.

12. Ahn SH, Kim JH, Lee SH, Kim WJ, Kim DH, Park YH, et al. Comparison of voice outcomes in early glottic cancer patients after laser surgery or radiotherapy. Korean J Otorhinolaryngol Head Neck Surg. 2012; Nov. 55(11):701–6.

13. Kono T, Saito K, Yabe H, Uno K, Ogawa K. Comparative multidimensional assessment of laryngeal function and quality of life after radiotherapy and laser surgery for early glottic cancer. Head Neck. 2016; Jul. 38(7):1085–90.

14. Sjogren EV, van Rossum MA, Langeveld TP, Voerman MS, van de Kamp VA, Friebel MO, et al. Voice outcome in T1a midcord glottic carcinoma: laser surgery vs radiotherapy. Arch Otolaryngol Head Neck Surg. 2008; Sep. 134(9):965–72.
15. Aaltonen LM, Rautiainen N, Sellman J, Saarilahti K, Makitie A, Rihkanen H, et al. Voice quality after treatment of early vocal cord cancer: a randomized trial comparing laser surgery with radiation therapy. Int J Radiat Oncol Biol Phys. 2014; Oct. 90(2):255–60.

16. Ma Y, Green R, Pan S, McCabe D, Goldberg L, Woo P. Long-term voice outcome following radiation versus laser microsurgery in early glottic cancer. J Voice. 2019; Mar. 33(2):176–82.

17. van Gogh CD, Verdonck-de Leeuw IM, Wedler-Peeters J, Langendijk JA, Mahieu HF. Prospective evaluation of voice outcome during the first two years in male patients treated by radiotherapy or laser surgery for T1a glottic carcinoma. Eur Arch Otorhinolaryngol. 2012; Jun. 269(6):1647–52.

18. Nunez Batalla F, Caminero Cueva MJ, Senaris Gonzalez B, Llorente Pendas JL, Gorriz Gil C, Lopez Llames A, et al. Voice quality after endoscopic laser surgery and radiotherapy for early glottic cancer: objective measurements emphasizing the Voice Handicap Index. Eur Arch Otorhinolaryngol. 2008; May. 265(5):543–8.

19. Krengli M, Policarpo M, Manfredda I, Aluffi P, Gambaro G, Panella M, et al. Voice quality after treatment for T1a glottic carcinoma—radiotherapy versus laser cordectomy. Acta Oncol. 2004; 43(3):284–9.
20. Tamura E, Kitahara S, Ogura M, Kohno N. Voice quality after laser surgery or radiotherapy for T1a glottic carcinoma. Laryngoscope. 2003; May. 113(5):910–4.

21. Wedman J, Heimdal JH, Elstad I, Olofsson J. Voice results in patients with T1a glottic cancer treated by radiotherapy or endoscopic measures. Eur Arch Otorhinolaryngol. 2002; Nov. 259(10):547–50.

22. Rosier JF, Gregoire V, Counoy H, Octave-Prignot M, Rombaut P, Scalliet P, et al. Comparison of external radiotherapy, laser microsurgery and partial laryngectomy for the treatment of T1N0M0 glottic carcinomas: a retrospective evaluation. Radiother Oncol. 1998; Aug. 48(2):175–83.

23. Rydell R, Schalen L, Fex S, Elner A. Voice evaluation before and after laser excision vs. radiotherapy of T1A glottic carcinoma. Acta Otolaryngol. 1995; Jul. 115(4):560–5.

24. McGuirt WF, Blalock D, Koufman JA, Feehs RS, Hilliard AJ, Greven K, et al. Comparative voice results after laser resection or irradiation of T1 vocal cord carcinoma. Arch Otolaryngol Head Neck Surg. 1994; Sep. 120(9):951–5.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download