INTRODUCTION
Sensorineural hearing loss is caused by the loss of the cochlear hair cells with the consequential degeneration of spiral ganglion neurons (SGNs), which relay auditory information from hair cells to the brainstem. The degeneration of SGNs is seen in circumstances such as noise exposure and aging, and overexposure to intense sound can cause an acute loss of SGN peripheral nerve terminals and a delayed loss of SGNs over a period of months [
1]. In a report studying the number of normal cochlear fibers in rat, their number in young adults (2–3 months) was reduced by 21% in adult (26.5 months) and 24% in oldest (35–36 months) mice [
2]. Degeneration of the SGNs following hair cell loss, which leaves fewer neurons available for electrical stimulation, carries critical implications for cochlear implants (CIs) because they stimulate the spiral ganglion cells directly by bypassing the cochlear hair cells.
Recent advances in stem cell therapy and cellular reprogramming have developed several possibilities to induce endogenous regeneration or exogenous stem cell transplantation for replacing decreased SGNs to enhance hearing restoration in patients with CIs [
3-
5]. However, the lack of suitable donor cells and the low survival rate of the implanted cells are major obstacles to the successful implementation of exogenous transplantation [
6]. Several studies have reported the identification of SGN-derived neural stem cells (SGN-NSCs) and their differentiation into neurons to replace damaged SGNs by the endogenous regeneration of the SGNs [
7-
10]. It was reported that SGNs from humans aged between 40 and 60 years old formed spheres that proliferated and differentiated into mature neurons and glial cells, but mouse studies have revealed the existence of sphere-forming SGN-NSCs only during the early postnatal period of up to 3 weeks [
8,
9]. Thus, it is still unclear how long the SGN-NSCs remain present in the cochlear modiolus.
In this study, we examined the expression of Nestin and Ki67 in sequentially dissected cochlear modiolar tissues from mice of different ages (from postnatal day [P1] to 24 weeks) as well as the isolation of sphere-forming stem cells from spiral ganglion cells and their differentiation into different cell types.
MATERIALS AND METHODS
Experimental animals and ethical considerations
The mice for the experiments were maintained on a C57BL/6 background. All of them were housed under a 12-hour light-dark cycle and had access to food and water ad libitum in a controlled animal facility. All of the animal experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Southern California (Protocol No. 11489) and the National Institute of Health.
The isolation, culture, and propagation of sphere-forming stem cells from the early and late postnatal spiral ganglion
The mice (P1, n>30; 2 weeks, n>30; 1 month, n>15; 3 month, n>10; 6 month, n>10) were decapitated, after which the otic capsule was dissected out after removal of the brain and immersed in ice-cold phosphate-buffered saline (PBS; Invitrogen, Carlsbad, CA, USA). The bony otic capsule was opened and removed to visualize the membranous labyrinth of the cochlea. The cochlear duct was microdissected from the modiolus where the spiral ganglion resides. The isolated spiral ganglion cells were cultured in N2 medium containing basic fibroblast growth factor (bFGF; 20 ng/mL, PeproTech, Rocky Hill, NJ, USA) for 4 days to maintain stem cell characteristics and then evaluated for Nestin and Sox2 expression. To induce differentiation, the cells were seeded and further cultured in bFGF depleted medium [
11-
13]. Neurosphere culturing was conducted as has been described previously [
11-
13].
The antibodies and reagents
The antibodies used in this study were anti-Ki67 (rabbit polyclonal 1:500; Abcam, Cambridge, MA, USA), anti-Nestin (mouse monoclonal 1:350; BD Biosciences, San Jose, CA, USA), anti-Sox2 (rabbit polyclonal 1:500, Abcam), anti-Tuj1 (mouse polyclonal 1:200; Covance, Princeton, NJ, USA), anti-MAP2 (rabbit polyclonal 1:200; Chemicon, Temecula, CA, USA), and anti-GFAP (rabbit 1:500; Cell Signaling Technology, Danvers, MA, USA). Secondary antibodies were anti-rabbit Alexa Fluor 488-conjugated, anti-mouse Alexa Fluor 488-conjugated, anti-rabbit Alexa Fluor 555-conjugated, and anti-mouse Alexa Fluor 555-conjugated immunoglobulin G (1:200 dilution; Molecular Probes, Eugene, OR, USA). The protease inhibitor cocktail was from Roche Applied Science (Indianapolis, IN, USA).
Immunohistochemistry and immunocytochemistry
Immunohistochemistry and immunocytochemistry was conducted as has been described previously [
11-
13]. Briefly, postnatal mice (P1, 2 weeks old) were euthanized with carbon dioxide. Cochlear tissues were isolated from the head and fixed in 4% paraformaldehyde at 4°C overnight. Adult mice (4 weeks, 12 weeks, and 24 weeks old) were anesthetized with isoflurane and transcardially perfused with saline, followed by 4% paraformaldehyde. Temporal bones containing the inner ear were removed and fixed in 4% paraformaldehyde overnight, after which they were moved to a 4% solution of ethylenediaminetetraacetic acid for 5 days to decalcify the bone. Cochlear tissues were cryoprotected in 30% sucrose, embedded and frozen in the Tissue Tek OCT compound, and sectioned at 20 μm on a cryostat. For the immunocytochemistry, spiral ganglion NSCs cultured on coverslips were fixed with 4% paraformaldehyde/PBS for 30 minutes and immunostained after permeabilization with 0.2% Triton X-100. The cryosections or cells grown on coverslips were incubated with one of the primary antibodies at 4ºC overnight. Cells and tissues were washed three times in PBS and incubated with Alexa Fluor 488- or 555-conjugated immunoglobulin G secondary antibody (Molecular Probes) at room temperature for 1 hour. Nuclei were counterstained in 1 μg/mL DAPI (4´, 6-diamidino-2-phenylindole; Boehringer Mannheim, Mannheim, Germany) for 10 minutes followed by exhaustive washing in distilled water. The images were visualized using confocal microscopy (LSM 700; Zeiss, Jena, Germany). For quantification of images, approximately 100 cells were counted in at least three randomly selected 400× fields, and the percentages of proliferating cells (Ki67- or Nestin-positive) and progenitor- and neural differentiation-marker expressing cells were determined. All data were presented as mean and standard deviation (SD).
The quantitative polymerase chain reaction protocol
The quantitative polymerase chain reaction (qPCR) was conducted as has been described previously [
11,
13]. The primer sets for the qPCR protocol are listed in
Supplementary Table 2.
Statistical analysis
Statistical differences among the groups were analyzed using the Student t-test or analysis of variance (Tukey’s multiple comparison test). The values obtained from at least three independent experiments were averaged and reported as the mean and SD. Any differences are indicated in the figures as follows: P<0.05, P<0.005, and P<0.0005. The P-value <0.05 was considered as statistically significant.
RESULTS
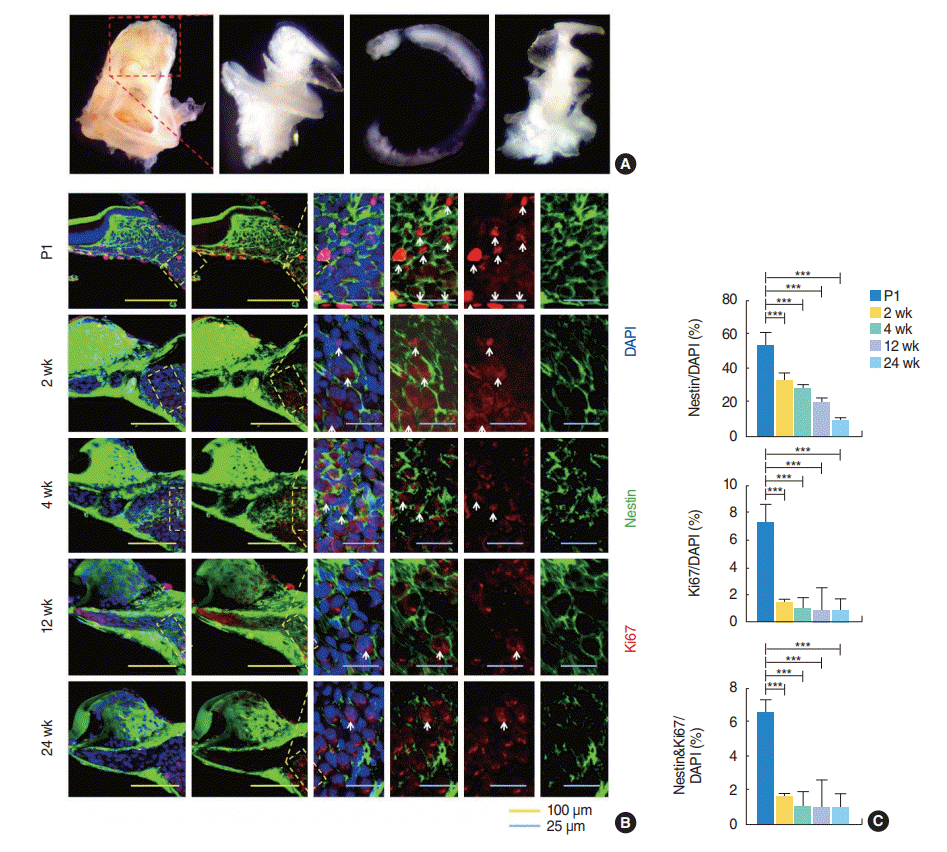
Nestin-positive proliferating spiral ganglion cells are present in both young and old adult cochlea. To characterize the SGN-NSCs presenting in the SGNs of mice of different ages
in vivo, we isolated the SGNs from the cochlear modiolus of mice of early (P1 and 2 weeks) to late (4, 12, and 24 weeks) age (
Fig. 1A). To characterize the number and localization of the SGN-NSCs, we undertook an immunohistochemical analysis of the cochlear tissues derived from the mice and observed a slight decrease in the number of cells positive for the NSC-marker Nestin (~52%, ~32%, ~27%, ~20%, and ~9% at P1, 2 weeks, 4 weeks, 12 weeks, and 24 weeks, respectively) with mouse age (
Fig. 1B,
C). Similarly, we observed a significant decrease in proliferation marker Ki-67-positive cells (~7.3%, ~1.5%, ~1%, ~1%, and ~0.9% at P1, 2 weeks, 4 weeks, 12 weeks, and 24 weeks, respectively) and Nestin and Ki-67 double-positive proliferating stem cells (~6.5%, ~1.5%, ~1%, ~1%, and ~0.9% at P1, 2 weeks, 4 weeks, 12 weeks, and 24 weeks, respectively) with mouse age (
Fig. 1B,
C,
Supplementary Table 1).
Fig. 1.
Surgical dissection of the cochlea and sections for spiral ganglion neurons in postnatal mice as a function of age. (A) The bony shell of the cochlea (red box) was removed and then spiral cochlear tissue was removed, resulting in the modiolus containing spiral ganglion neurons. (B) Fixed coronal sections of the spiral ganglion neurons (yellow boxes) stained with antibodies against Nestin (green) and Ki67 (red). White arrows indicate Nestin and Ki67 double positive cells. (C) Quantification of the data shown in (B) (postnatal day 1 [P1], n=4; 2 weeks, n=3; 4 weeks, n=3; 12 weeks, n=3; and 24 weeks, n=3). All individual quantification data underlying (C) can be found in
Supplementary Table 1. Values are presented as mean±standard deviation. Analysis of variance tests were performed to calculate significance (***
P<0.0001). DAPI, 4´,6-diamidino-2-phenylindole.

SGNs contain self-renewing and proliferative activity
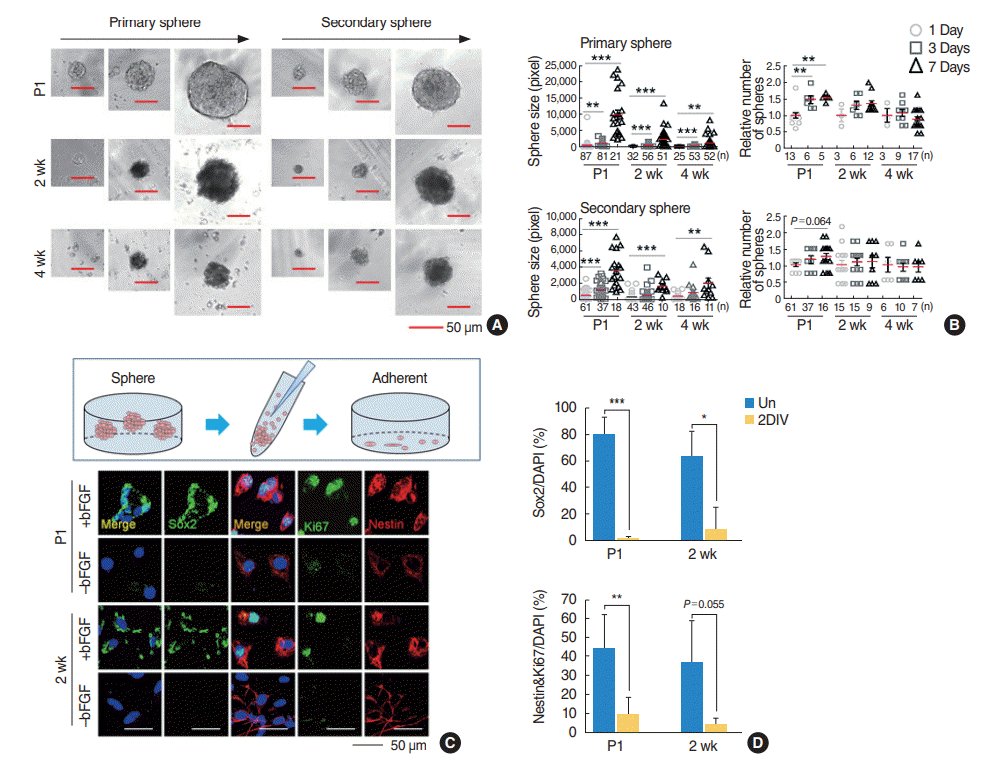
Subsequently, to compare the biological significance of the SGN-NSCs derived from the cochlea of mice of different ages, we examined their self-renewing capacity by performing a sphere-forming assay in which the dissociated SGN-NSCs formed spheres at 1, 3, and 7 days in a serum-free medium (
Fig. 2A). The dissociated cells from the primary spheres were grown further to form secondary spheres which showed self-renewing ability. The size and number of spheres in the P1 mice SGN-NSCs increased significantly at 1, 3, and 7 days although this activity decreased critically in the 2- and 4-week mice SGN-NSCs compared to the P1 mice SGN-NSCs (
Fig. 2A,
B). Moreover, to assess the stemness and proliferation of the SGN-NSCs, we successfully cultured both the P1 and P2-week-old mice SGN-NSCs in the presence of bFGF, but the number of SGN-NSCs from P4-week-old mice was not enough for immunocytochemical analysis. Sox2-positive populations were highly expressed (~80% and ~60% in the P1 and P2-week-old mice SGN-NSCs, respectively) and significantly decreased in both sets of cells over the course of differentiation (
Fig. 2C,
D). We also observed a high percentage of Nestin and Ki67 double-positive cells (~45% of SGN-NSCs in the P1 mice and ~35% in the P2-week-old mice) which significantly decreased over the course of differentiation in both sets of cells.
Fig. 2.
Spiral ganglion cells exhibit self-renewing and proliferative activity. (A) Spiral ganglions were separated from mice of age postnatal day 1 (P1), 2 weeks, and 4 weeks, and sphere formation was induced for 7 days in vitro. (B) Quantification of the relative sizes and numbers of primary or secondary spheres in (A). The number of P1 spiral ganglion neuron-derived neural stem cell spheres formed during 1 day culture was normalized to 1. Values are presented as mean±standard deviation. Analysis of variance tests were performed to calculate significance (*P<0.01, **P<0.001, ***P<0.0001). (C) P1 spiral ganglion cells dissociated from the spheres (upper) were grown and were subjected to immunofluorescence labeling using antibodies specific for Sox2 (green), Ki67 (green), and Nestin (red). (D) Quantification of anti-Sox2-positive (P1: +bFGF, n=3; –bFGF, n=5; 2 weeks: +bFGF, n=3; –bFGF, n=4), or anti-Nestin and anti-Ki67 double-positive cells (P1: +bFGF, n=6; –bFGF, n=5; 2 weeks: +bFGF, n=4; –bFGF, n=3) in (C). The t-tests were performed to calculate significance (*P<0.05, **P<0.005, ***P<0.0005). bFGF, basic fibroblast growth factor; DAPI, 4´,6-diamidino-2-phenylindole; Un, undifferentiated; 2DIV, 2 days differentiation in vitro.

SGNs contain multipotent differentiation potentials
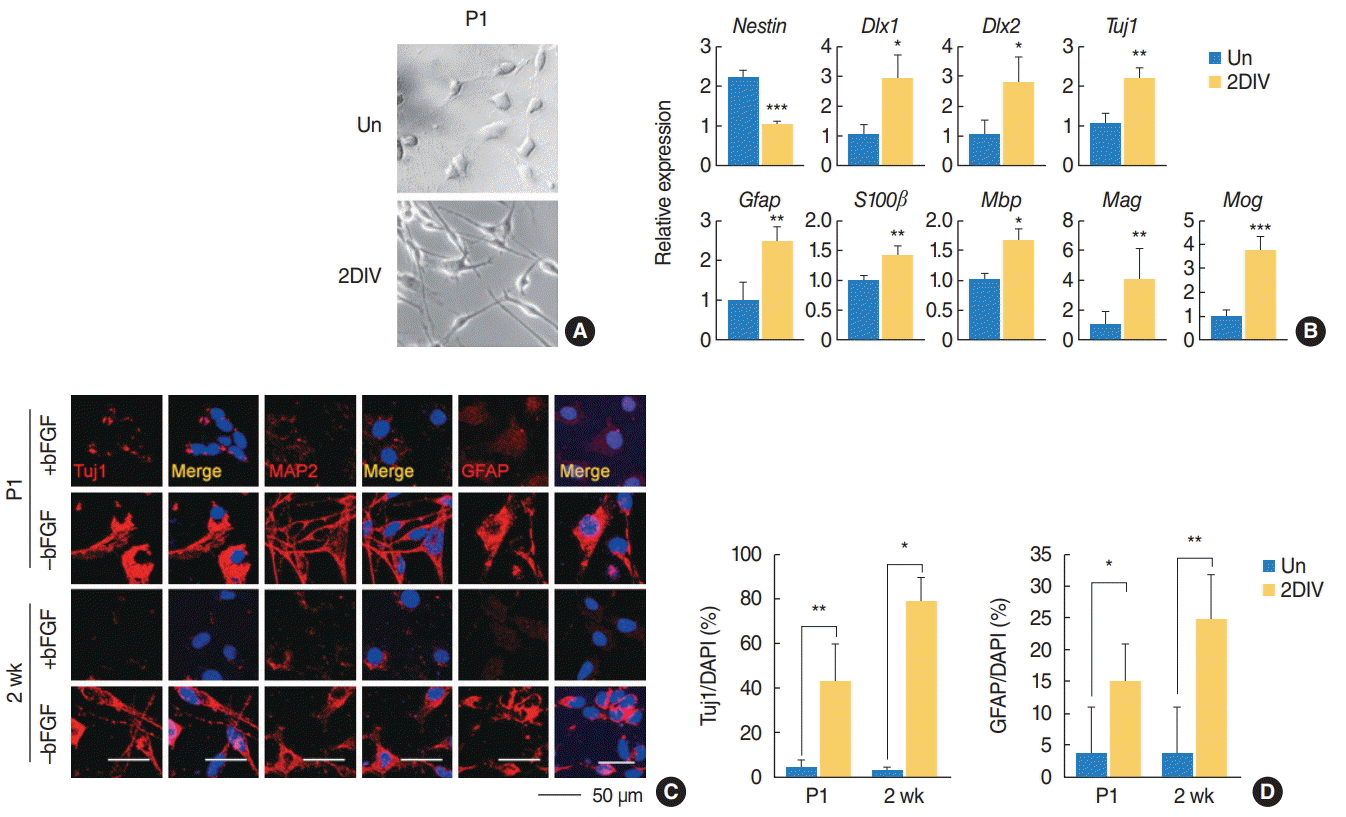
We also tested the differentiation potential of the SGN-NSCs in mice of different ages by observing their morphological changes (
Fig. 3A). Upon induction of differentiation, the neurite outgrowth of the SGN-NSCs increased significantly (
Fig. 3A). Further analysis using qPCR showed that the SGN-NSCs exhibited increased expression of
Dlx1, Dlx2, and
Tuj1 (markers for neuronal lineage) as well as other neural differentiation genes such as
Gfap, S100β (an astrocyte marker),
Mbp, Mog, and
Mag (the latter being an oligodendrocyte marker), and decreased expression of
Nestin (
Fig. 3B). Moreover, an immunocytochemical analysis of the P1 and P2-week-old mice SGN-NSCs revealed an increase in the number of cells positive for Tuj1, mature microtubule-associated protein 2 (MAP2), or GFAP (
Fig. 3C,
D). These results indicate that the SGN-NSCs attained a multipotent capacity.
Fig. 3.
The spiral ganglion cells exhibited a multipotent capacity. (A) Spiral ganglions were grown and their differentiation capabilities monitored by changes in morphology. (B) Quantitative polymerase chain reaction analysis of the indicated mRNA sequences in the postnatal day 1 (P1) mice spiral ganglions (n=4). Values are presented as mean±standard deviation. (C) Immunostaining with Tuj1, MAP2, and GFAP (all red) antibodies in P1 and 2-week-old mice under undifferentiation and differentiation conditions. (D) Quantification of Tuj1-positive (P1: +bFGF, n=3; –bFGF, n=5; 2 weeks: +bFGF, n=3; –bFGF, n=4) and GFAP-positive cells (P1: +bFGF, n=5; –bFGF, n=5; 2 weeks: +bFGF, n=4; –bFGF, n=4) in (C). The t-tests were conducted to calculate the statistical significance (*P<0.05, **P<0.005, ***P<0.0005). Un, undifferentiated; 2DIV, 2 days differentiation in vitro; bFGF, basic fibroblast growth factor; MAP2, mature microtubule-associated protein 2; DAPI, 4´,6-diamidino-2-phenylindole.

The spiral ganglion consists of heterogeneous populations and the SGN-NSCs are a regenerative source for repairing damaged cells
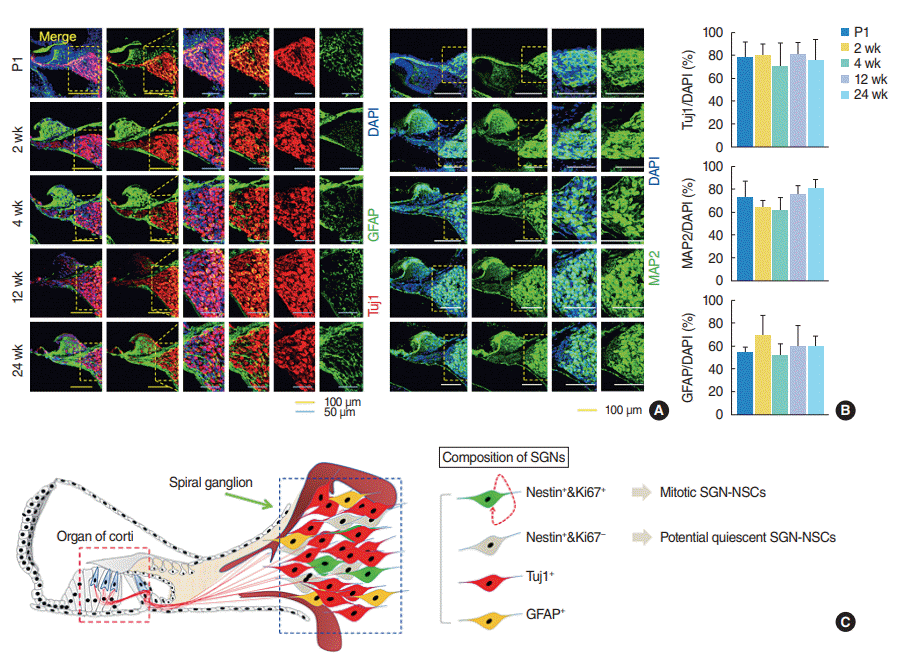
To characterize the neural connections and glial cell composition in the spiral ganglion modiolus further, we undertook an immunohistochemical analysis of the cochlear tissues derived from the mice of different ages and observed the number of immature Tuj1-positive and mature MAP2-positive neurons, or GFAP-positive astrocytes did not significantly change in the mice of different ages (
Fig. 4A,
B). Overall, the spiral ganglion was heterogeneous and consisted of multiple populations of neuron, glial, non-proliferative SGNs, and proliferative SGN-NSCs which exhibited both self-renewing activity and a multipotent capacity (
Fig. 4C).
Fig. 4.
Spiral ganglion cells consist of heterogeneous cell populations, of which spiral ganglion neuron-derived neural stem cell spheres (SGNNSCs) could be a regenerative source for damaged cell repair. (A) Fixed coronal sections of the cochlea stained with antibodies against GFAP (green), Tuj1 (red), and MAP2 (green). (B) Quantification of Tuj1-positive (postnatal day 1 [P1], n=4; 2 weeks, n=4; 4 weeks, n=3; 12 weeks, n=4; 24 weeks, n=4), MAP2-positive (P1, n=4; 2 weeks, n=4; 4 weeks, n=4; 12 weeks, n=4; 24 weeks, n=4), and GFAP-positive (P1, n=4; 2 weeks, n=4; 4 weeks, n=3; 12 weeks, n=4; 24 weeks, n=4), in (A). (C) Schematic showing the existence of regenerative and self-renewing SGN-NSCs. DAPI, 4´,6-diamidino-2-phenylindole; MAP2, mature microtubule-associated protein 2.

DISCUSSION
The existence of SGN-NSCs with stem cell-like properties have been confirmed in culture systems in vitro using ganglion cells derived from the cochlear modiolus of adult mice, but it is unclear how many SGN-NSCs were present and their age dependency in vivo. Given the significance of human SGN-NSCs as a potential source for regeneration and restoring hearing loss after cochlear implantation, further investigation was necessary to show the number of SGN-NSCs present in mice of different ages.
We observed significant decreases in Nestin and Ki-67 double-positive proliferating cells (~6.5%, ~1.5%, ~1%, ~1%, and ~0.9% at P1, 2 weeks, 4 weeks, 12 weeks, and 24 weeks, respectively) in vivo as a function of mouse age. Moreover, we observed a higher percentage of cells positive for the NSC marker Nestin (~52%, ~32%, ~27%, ~20%, and ~9% at P1, 2 weeks, 4 weeks, 12 weeks, and 24 weeks, respectively). These results demonstrate that in mice of different ages, the number of Nestin-positive SGN-NSCs was maintained to some extent, while the number of proliferating SGN-NSCs decreased, suggesting that a reduction in the regenerative ability in older adults compared to younger adults may be associated with a decrease in proliferating SGN-NSCs. Further, Nestin-positive and Ki67-negative cells need to be further characterized for their quiescent NSC property and potential to re-enter cell cycle. If they retain the ability to re-enter cell cycle and form functional SGNs, the availability of 9% Nestin-positive cells towards late age of 24 weeks holds huge promise for regeneration even in adults.
Our findings demonstrated the presence of SGN-NSCs in the mouse cochlear modiolus both
in vitro and
in vivo. Nestin-positive SGN-NSCs grew as neurospheres over time in culture and proliferated, as shown by the Ki67 labeling and the subculturing of the spheres. Although mammalian SGNs do not exhibit significant regenerative capacity, several groups have demonstrated that growth factor responsive progenitor/stem cells that proliferate and differentiate into mature neurons and glia cells could be isolated from the SGNs even in old adults [
6,
8,
9], which is in agreement with our findings. Furthermore, it has been reported that mitotic cells were found by means of the BrdU (5-bromo- 2’-deoxyuridine) incorporation method postnatally (P14) [
14], although it was reported that the mitosis of mouse SGNs has mainly completed by gestational day 16 (E16) [
15]. Our results also showed the presence of a few number of proliferated cells in mice of ages 12 weeks and older as indicated by Ki-67 marker. It was reported that Ki-67 antigen is expressed in all cycling cells, whereas BrdU reflects only the S-phage cells [
16]. Importantly, the amount of proliferative stem cell populations shown in the P1 and 2-week-old mice SGN-NSCs were higher than those seen in the 4-, 12-, and 24-week-old mice SGN-NSCs, which supports the idea that the SGN-NSC spheres derived at an early age in mice contain more mitotic stem cells than those in mice at a later age. In immunohistochemical analysis with cochlear tissues derived from 12- and 24-week-old mice, we observed proliferative Nestin and Ki-67 double positive cells, but we couldn’t succeed to grow SGN-NSCs and their spheres
in vitro analysis. This might be due to low number of proliferative SGN-NSC population in old mice and further studies should be addressed. The inability to increase sphere size and number with age suggests that cells would potentially be entering a quiescent cell state due to intrinsic properties of stem cell to create a reserve pool once organ development is complete. Further studies are required to identify extrinsic molecules that can rewire this intrinsic cell property and allow the stem cells to proliferate in a homeostatic manner.
In our experiments, cell-type-specific antibodies showed their multipotency by the presence of Tuj1-positive early neurons, MAP2-positive matured neurons, and GFAP-positive astrocytes, which is similar to previous reports [
8,
9]. In our SGN-NSCs cultures, we observed Nestin-positive NSC populations that divided over time, as shown by the Ki-67 staining and the secondary subcultivation of the neurospheres. These cells subsequently differentiated into neurons in the absence of bFGF. Therefore, our conclusion is that the SGN dividing cells, rather than being differentiated mature neurons or glial cells, represent proliferating NSCs that exist in the mature spiral ganglia of the VIII cranial nerve.
Previous mouse studies have revealed the existence of sphere-forming SGN-NSCs from embryonic day 12.5 to postnatal day 5 [
6,
7,
10]. In another study, the spiral ganglion was shown to possess a high proliferative potential after birth but to lose the vast majority of stem cells steeply between the second and third postnatal week, and to show no proliferative activity at P42 [
8]. It has been reported that SGN-NSCs exist even in human adults aged between 40 and 60 years old and develop into the form of spheres that proliferate and differentiate into mature neurons and glial cells [
9], suggesting that these cells could be of importance for the self-repair, self-renewing, and maintenance of the functional integrity of the auditory nerve throughout life [
17]. We observed that the proliferative potential of the SGN-NSCs in mice persisted up to postnatal 3–6 months
in vivo, albeit at a low level, suggesting that they might serve as donor cells in stem cell-based SGN replacement therapy, even at a later age in the animals’ lives.
SGNs are an essential functional component of the peripheral auditory system. Most types of hearing loss are associated with spiral ganglion cell degeneration, which is irreversible due to the inner ear’s lack of regenerative capacity. Although transplantation of exogenous stem cells to replace or maintain decreased SGNs has been pursued to restore hearing function [
4,
18-
20], the lack of functional and survived donor cells after implantation of cells are major issues to the successful implementation of therapeutic transplantation [
6]. Another option is to recruit new SGNs from endogenous cell sources with functional connections in the auditory system under appropriate growth factor stimulation [
3]. If there are NSCs in the SGN region, the lost SGNs might be replaced by NSCs. It has been reported that embryonic stem cells and SGNs from newborn mice make synapses with hair cells and contact neurons in the brainstem [
4,
7,
21]. Poor prognostic factors for CI outcomes, such as old age and a long duration of deafness, in postlingually deaf adults are possibly related to a decrease in SGN count, which leaves fewer neurons available for stimulation by the CI, even though central re-organization might also play a role in determining the CI outcomes [
22-
24]. The advantage of CI on the survival of SGNs is that electrical stimulation could enhance their survival and a combination of induced expression of glial cell line-derived neurotrophic factor in the cochlea using viral vectors and electrical stimulation has also been shown to have a synergic effect on increasing the survival of SGNs [
25,
26], leading to the better survival or regeneration of SGNs from endogenous NSCs in patients with a CI for hearing restoration.
In summary, we found the presence of sphere-forming stem cells isolated from the spiral ganglion of postnatal mice both
in vivo and
in vitro, although the proliferating activity decreased significantly with age. We showed that spiral ganglion spheres have characteristics similar to neurospheres isolated from the brain and maintained their major stem cell characteristics after repeated propagation [
11-
13], which enabled the culturing of spheres over an extended period of time. The SGN-NSCs could differentiate into several cell populations, including neurons and glial cells. Thus, our work provides evidence for the presence of self-renewing spiral ganglion stem cells which might serve as a promising source for the regeneration of lost auditory neurons.








 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download