INTRODUCTION
MATERIALS AND METHODS
Experimental design
Cochlear ablation
ABR recordings
Western blot assay
Statistical analysis
RESULTS
Time course of auditory-somatosensory plasticity following hearing loss
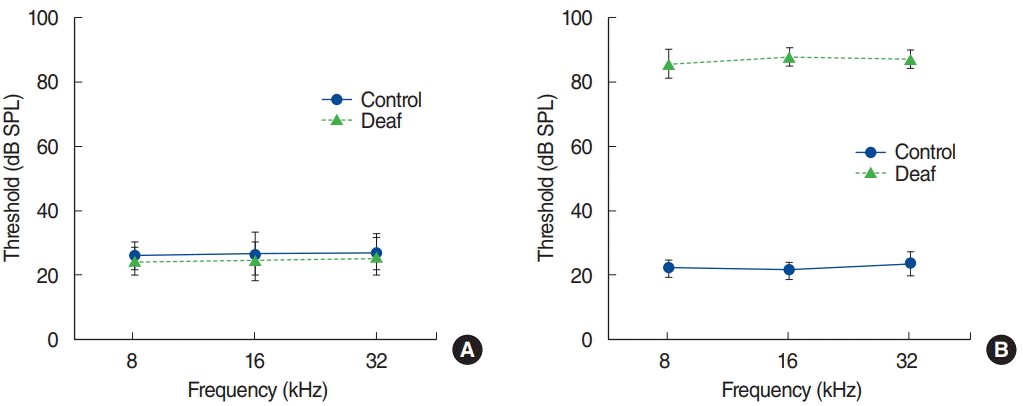
ABR recordings
Disruption of the VGLUT2/VGLUT1 ratio after hearing loss
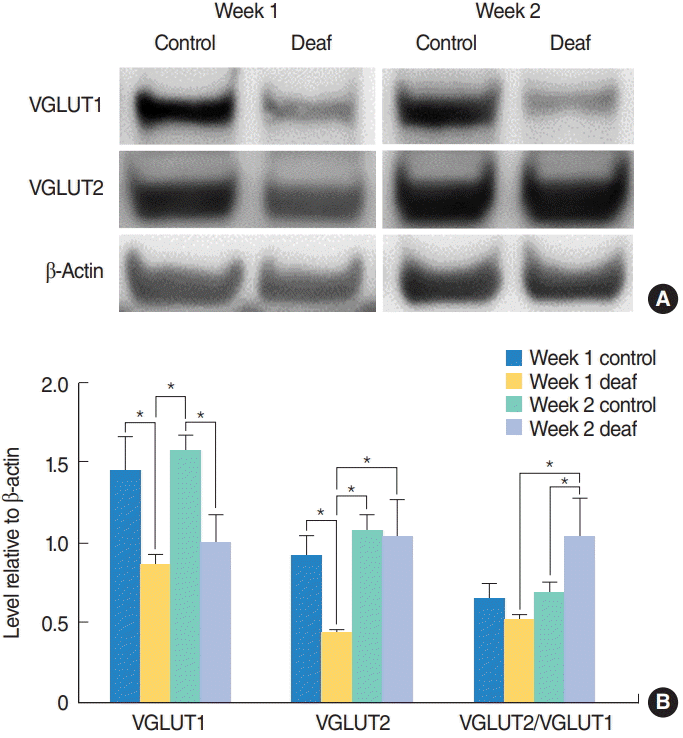 | Fig. 2.Increase in the VGLUT2/VGLUT1 ratio after hearing loss. VGLUT1 and VGLUT2 expression levels in the cochlear nucleus were examined with a Western blot assay 1 and 2 weeks after surgery. (A) Representative Western blots. (B) Quantitative analysis of VGLUT1 and VGLUT2. At 2 weeks after surgery, the deaf group had a significantly higher VGLUT2/VGLUT1 ratio compared to the control group. Values are presented as mean±standard error. VGLUT1, vesicular glutamate transporter 1; VGLUT2, vesicular glutamate transporter 2. *Statistically significant differences between groups, P<0.05. |
Prevention of auditory-somatosensory plasticity using losartan
ABR recordings
Prevention of disruption of the VGLUT2/VGLUT1 ratio
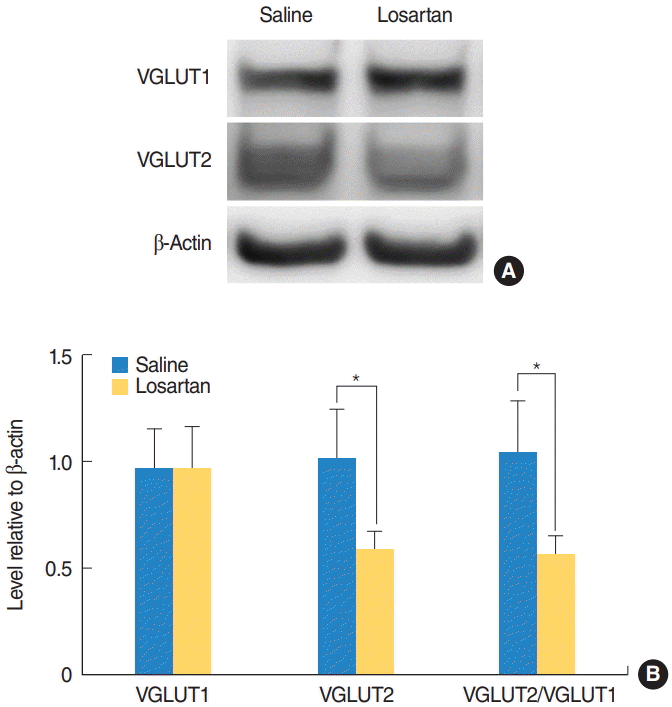 | Fig. 3.Prevention of the increase in the VGLUT2/VGLUT1 ratio using losartan. VGLUT1 and VGLUT2 expression levels in the cochlear nucleus were examined with a Western blot assay 2 weeks after surgery. (A) Representative Western blots. (B) Quantitative analysis of VGLUT1 and VGLUT2. The losartan group had a significantly lower VGLUT2/VGLUT1 ratio compared to the deaf group. Values are presented as mean±standard error. VGLUT1, vesicular glutamate transporter 1; VGLUT2, vesicular glutamate transporter 2. *Statistically significant differences between groups, P<0.05. |
p-Smad2/3 signaling and GAP-43
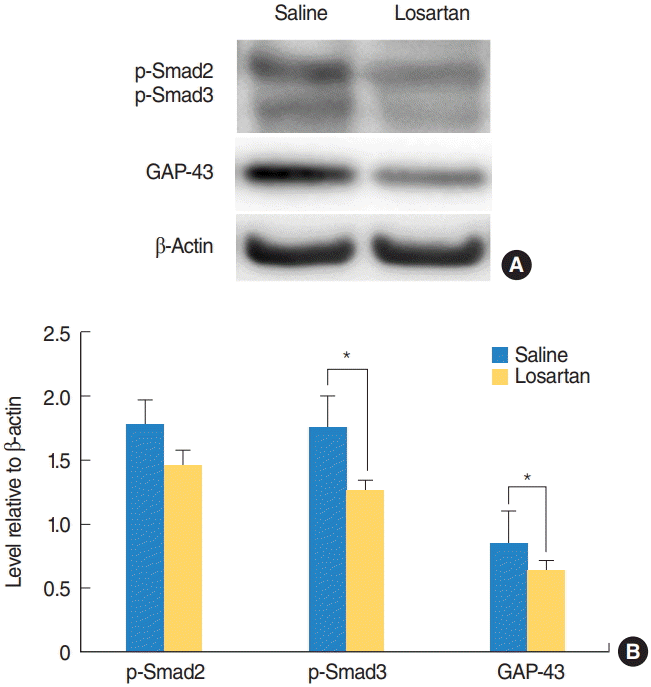 | Fig. 4.Changes in p-Smad2 and 3 signaling, and GAP-43 following losartan administration. p-Smad2 and 3, and GAP-43 expression levels in the cochlear nucleus were examined with a Western blot assay 2 weeks after surgery. (A) Representative Western blots. (B) Quantitative analysis of p-Smad2 and 3, and GAP-43. The losartan group had significantly lower p-Smad3 and GAP-43 levels compared to the deaf group. Values are presented as mean±standard error. GAP-43, growth-associated protein 43. *Statistically significant differences between groups, P<0.05. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download