1. Zang M, Zhang Q, Chang EI, Mathur AB, Yu P. Decellularized tracheal matrix scaffold for tissue engineering. Plast Reconstr Surg. 2012; Sep. 130(3):532–40.

2. Macchiarini P, Walles T, Biancosino C, Mertsching H. First human transplantation of a bioengineered airway tissue. J Thorac Cardiovasc Surg. 2004; Oct. 128(4):638–41.

3. Go T, Jungebluth P, Baiguero S, Asnaghi A, Martorell J, Ostertag H, et al. Both epithelial cells and mesenchymal stem cell-derived chondrocytes contribute to the survival of tissue-engineered airway transplants in pigs. J Thorac Cardiovasc Surg. 2010; Feb. 139(2):437–43.

4. Jungebluth P, Moll G, Baiguera S, Macchiarini P. Tissue-engineered airway: a regenerative solution. Clin Pharmacol Ther. 2012; Jan. 91(1):81–93.

5. Benders KE, van Weeren PR, Badylak SF, Saris DB, Dhert WJ, Malda J. Extracellular matrix scaffolds for cartilage and bone regeneration. Trends Biotechnol. 2013; Mar. 31(3):169–76.

6. Schwarz S, Elsaesser AF, Koerber L, Goldberg-Bockhorn E, Seitz AM, Bermueller C, et al. Processed xenogenic cartilage as innovative biomatrix for cartilage tissue engineering: effects on chondrocyte differentiation and function. J Tissue Eng Regen Med. 2015; Dec. 9(12):E239–51.

7. Schwarz S, Koerber L, Elsaesser AF, Goldberg-Bockhorn E, Seitz AM, Dürselen L, et al. Decellularized cartilage matrix as a novel biomatrix for cartilage tissue-engineering applications. Tissue Eng Part A. 2012; Nov. 18(21-22):2195–209.

8. Yang Z, Shi Y, Wei X, He J, Yang S, Dickson G, et al. Fabrication and repair of cartilage defects with a novel acellular cartilage matrix scaffold. Tissue Eng Part C Methods. 2010; Oct. 16(5):865–76.

9. Kalathur M, Baiguera S, Macchiarini P. Translating tissue-engineered tracheal replacement from bench to bedside. Cell Mol Life Sci. 2010; Dec. 67(24):4185–96.

10. Sutherland AJ, Beck EC, Dennis SC, Converse GL, Hopkins RA, Berkland CJ, et al. Decellularized cartilage may be a chondroinductive material for osteochondral tissue engineering. PLoS One. 2015; May. 10(5):e0121966.

11. Baiguera S, Jungebluth P, Burns A, Mavilia C, Haag J, De Coppi P, et al. Tissue engineered human tracheas for in vivo implantation. Biomaterials. 2010; Dec. 31(34):8931–8.

12. Jungebluth P, Go T, Asnaghi A, Bellini S, Martorell J, Calore C, et al. Structural and morphologic evaluation of a novel detergent-enzymatic tissue-engineered tracheal tubular matrix. J Thorac Cardiovasc Surg. 2009; Sep. 138(3):586–93.

13. Remlinger NT, Czajka CA, Juhas ME, Vorp DA, Stolz DB, Badylak SF, et al. Hydrated xenogeneic decellularized tracheal matrix as a scaffold for tracheal reconstruction. Biomaterials. 2010; May. 31(13):3520–6.

14. Gilbert TW. Strategies for tissue and organ decellularization. J Cell Biochem. 2012; Jul. 113(7):2217–22.

15. Suzuki T, Kobayashi K, Tada Y, Suzuki Y, Wada I, Nakamura T, et al. Regeneration of the trachea using a bioengineered scaffold with adipose-derived stem cells. Ann Otol Rhinol Laryngol. 2008; Jun. 117(6):453–63.

16. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997; Apr. 276(5309):71–4.

17. Kafienah W, Mistry S, Williams C, Hollander AP. Nucleostemin is a marker of proliferating stromal stem cells in adult human bone marrow. Stem Cells. 2006; Apr. 24(4):1113–20.

18. Tsutsumi S, Shimazu A, Miyazaki K, Pan H, Koike C, Yoshida E, et al. Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem Biophys Res Commun. 2001; Oct. 288(2):413–9.

19. Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006; Jul. 27(19):3675–83.

20. Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011; Apr. 32(12):3233–43.

21. Hodde J, Hiles M. Virus safety of a porcine-derived medical device: evaluation of a viral inactivation method. Biotechnol Bioeng. 2002; Jul. 79(2):211–6.

22. Yang M, Chen CZ, Wang XN, Zhu YB, Gu YJ. Favorable effects of the detergent and enzyme extraction method for preparing decellularized bovine pericardium scaffold for tissue engineered heart valves. J Biomed Mater Res B Appl Biomater. 2009; Oct. 91(1):354–61.

23. Meezan E, Hjelle JT, Brendel K, Carlson EC. A simple, versatile, nondisruptive method for the isolation of morphologically and chemically pure basement membranes from several tissues. Life Sci. 1975; Dec. 17(11):1721–32.

24. Batioglu-Karaaltin A, Karaaltin MV, Ovali E, Yigit O, Kongur M, Inan O, et al. In vivo tissue-engineered allogenic trachea transplantation in rabbits: a preliminary report. Stem Cell Rev. 2015; Apr. 11(2):347–56.

25. Nireesha GR, Divya L, Sowmya C, Venkateshan N, Babu MN, Lavakumar V. Lyophilization/freeze drying: an review. Int J Nov Trends Pharm Sci. 2013; Oct. 3(4):87–98.
26. Wang S, Goecke T, Meixner C, Haverich A, Hilfiker A, Wolkers WF. Freeze-dried heart valve scaffolds. Tissue Eng Part C Methods. 2012; Jul. 18(7):517–25.

27. Hung SH, Su CH, Lee FP, Tseng H. Larynx decellularization: combining freeze-drying and sonication as an effective method. J Voice. 2013; May. 27(3):289–94.

28. Ingavle GC, Frei AW, Gehrke SH, Detamore MS. Incorporation of aggrecan in interpenetrating network hydrogels to improve cellular performance for cartilage tissue engineering. Tissue Eng Part A. 2013; Jun. 19(11-12):1349–59.

29. Roberts CR, Rains JK, Pare PD, Walker DC, Wiggs B, Bert JL. Ultrastructure and tensile properties of human tracheal cartilage. J Biomech. 1998; Jan. 31(1):81–6.

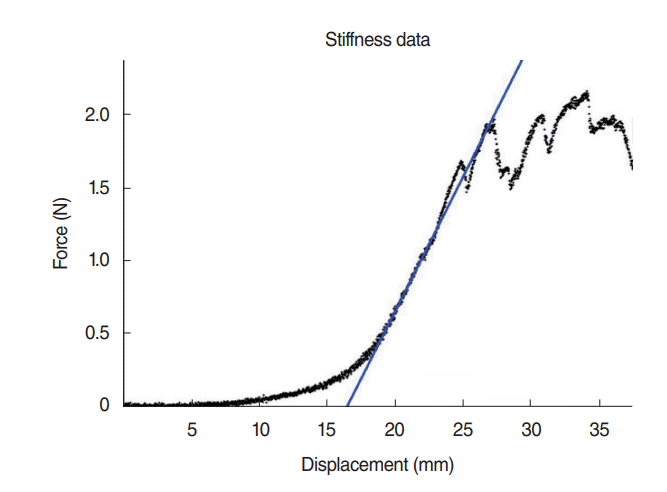
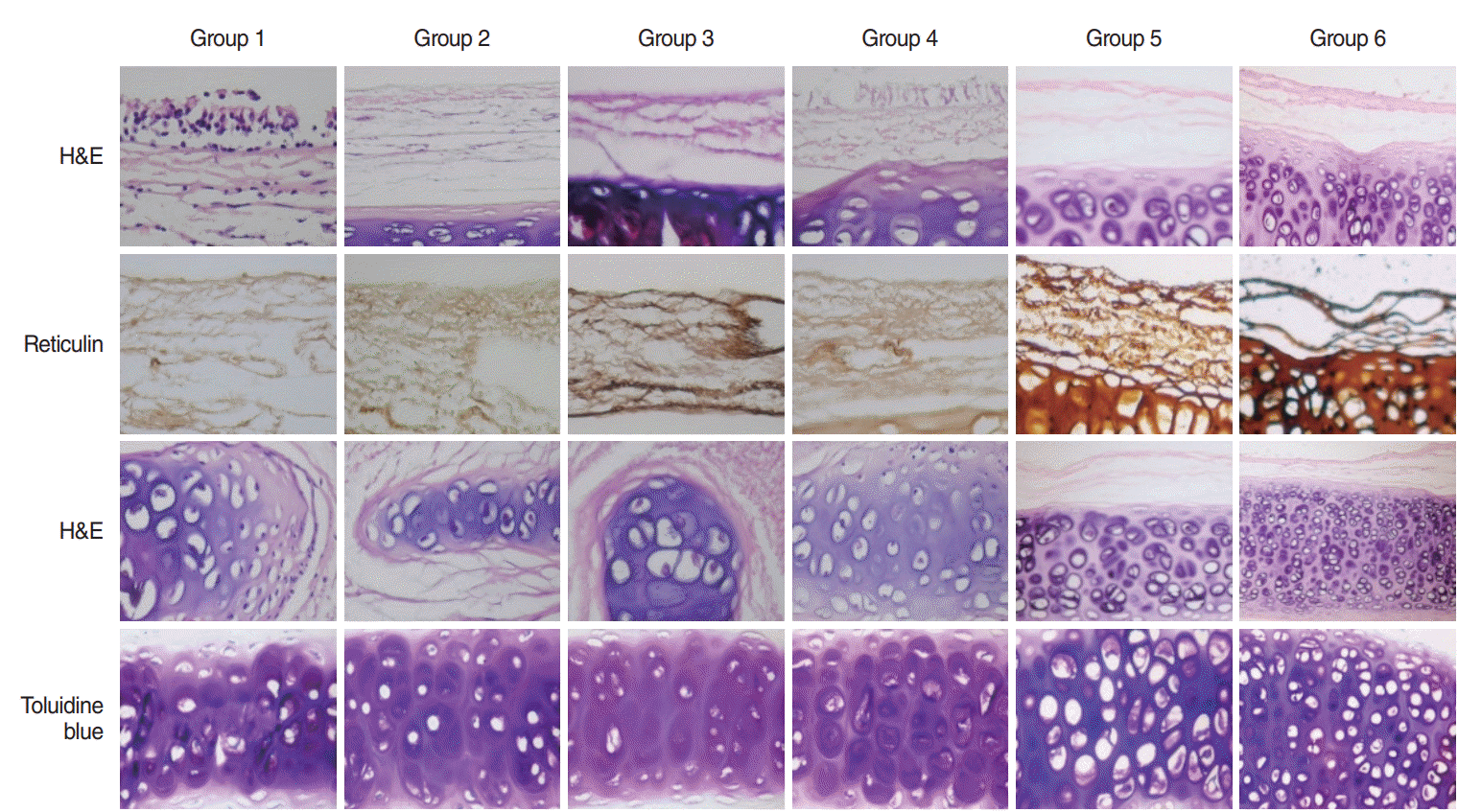
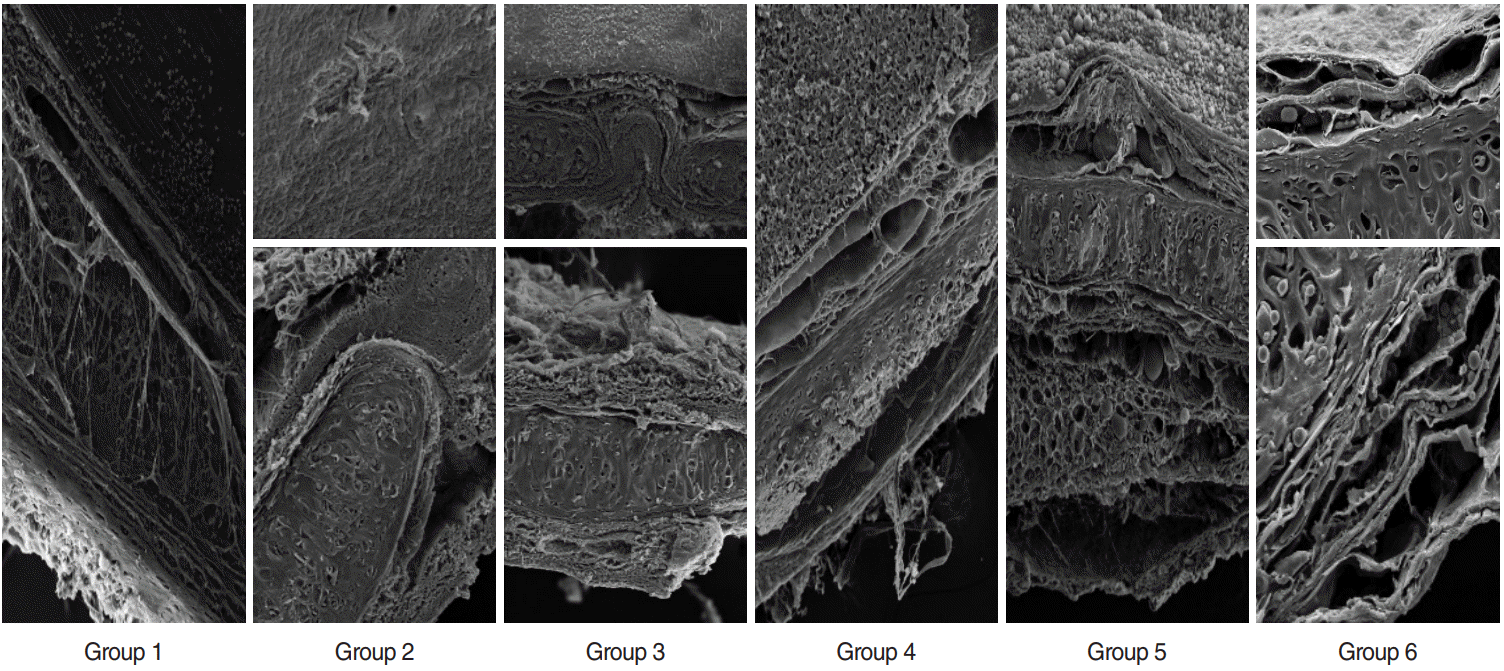




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download