INTRODUCTION
MATERIALS AND METHODS
Materials
Cell culture and treatment
RT-PCR of the mRNA expressions of MUC5AC, MUC5B, spliced XBP-1, CHOP, and ATF6
Table 1.
Real-time PCR for the mRNA expressions of MUC5AC, MUC5B, spliced XBP-1, CHOP, and ATF6
Enzyme-linked immunosorbent assay analysis of MUC5AC and MUC5B glycoproteins
Western blot analysis for XBP-1, CHOP, and ATF6
Cell transfection with siRNAs for XBP-1, CHOP, or ATF6
Statistical analysis
RESULTS
ER stress increased the expressions of MUC5AC and MUC5B in HNEpCs
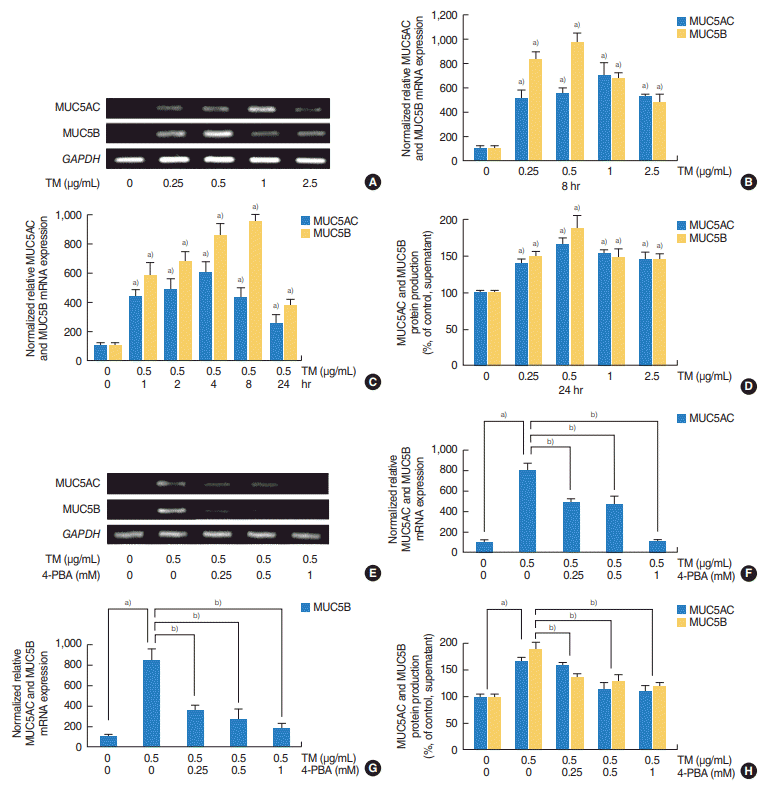 | Fig. 1.Effects of tunicamycin (TM; an endoplasmic reticulum stress inducer) on the expressions of MUC5AC and MUC5B in human nasal epithelial cells. (A, B) Reverse transcriptase-polymerase chain reaction (RT-PCR) and real-time PCR showed that TM significantly increased the mRNA expressions of MUC5AC and MUC5B at all concentrations examined. (C) Time-dependent changes, real-time PCR showed that TM induced the expressions of MUC5AC and MUC5B mRNA at all times. However, peak times differed; MUC5AC peaked after 4 hours of exposure to TM, whereas MUC5B peaked after 8 hours of exposure. (D) Enzyme-linked immunosorbent assay (ELISA) showed TM induced the productions of MUC5AC and MUC5B glycoproteins. Effects of 4-phenylbutyric acid (4-PBA; an endoplasmic reticulum stress inhibitor) on the TM-induced expressions of MUC5AC and MUC5B in human nasal epithelial cells. (E-G) RT-PCR and real-time PCR showed that 4-PBA significantly attenuated the TM-induced expressions of MUC5AC and MUC5B mRNA. (H) ELISA also showed that 4-PBA significantly attenuated TM-induced the productions of MUC5AC and MUC5B glycoprotein. The images shown are representative of three separate experiments performed in triplicate. Bars represent the averages±standard deviation of three independent experiments performed in triplicate. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. a)P<0.05 vs. baseline. b)P<0.05 vs. TM (0.5 µg/mL) alone. |
4-PBA reduced tunicamycin-induced MUC5AC and MUC5B expressions in HNEpCs
The three major signaling mechanisms were involved in the ER stress-induced expressions of MUC5AC and MUC5B in HNEpCs
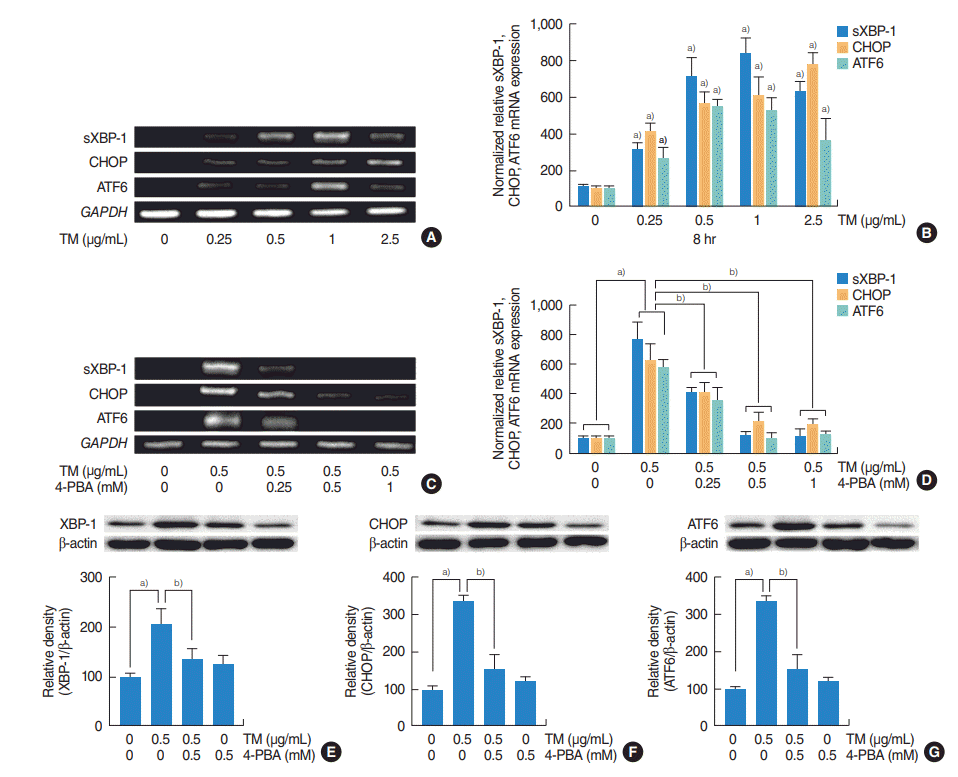 | Fig. 2.The roles of the three major endoplasmic reticulum stress-mediated signaling pathways (IRE1/XBP-1, PERK/ATF4/CHOP, and ATF6) on the expressions of MUC5AC and MUC5B in human nasal epithelial cells. (A-D) Reverse transcriptase-polymerase chain reaction (RT-PCR) and real-time PCR showed that the mRNA expressions of sXBP-1, CHOP, or ATF6 were significantly increased at all doses of tunicamycin (TM; 0.25, 0.5, 1, 2.5 µg/mL) administered and that these increases were attenuated by 4-phenylbutyric acid (4-PBA). (E-G) Western blot also showed that TM induced XBP-1, CHOP and ATF6 and attenuated by 4-PBA. The images shown are representative of three separate experiments performed in triplicate. Bars represent the averages±standard deviation of three independent experiments performed in triplicate. sXBP-1, spliced X-box binding protein 1; CHOP, CCAAT-enhancer-binding protein homologous protein; ATF6, activating transcription factor 6; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; XBP-1, X-box binding protein 1; IRE1, inositol requiring enzyme 1; PERK, RNA-activated protein kinase-like endoplasmic reticulum kinase; ATF4, activating transcription factor 4. a)P<0.05 vs. baseline. b)P<0.05 vs. TM (0.5 µg/mL) alone. |
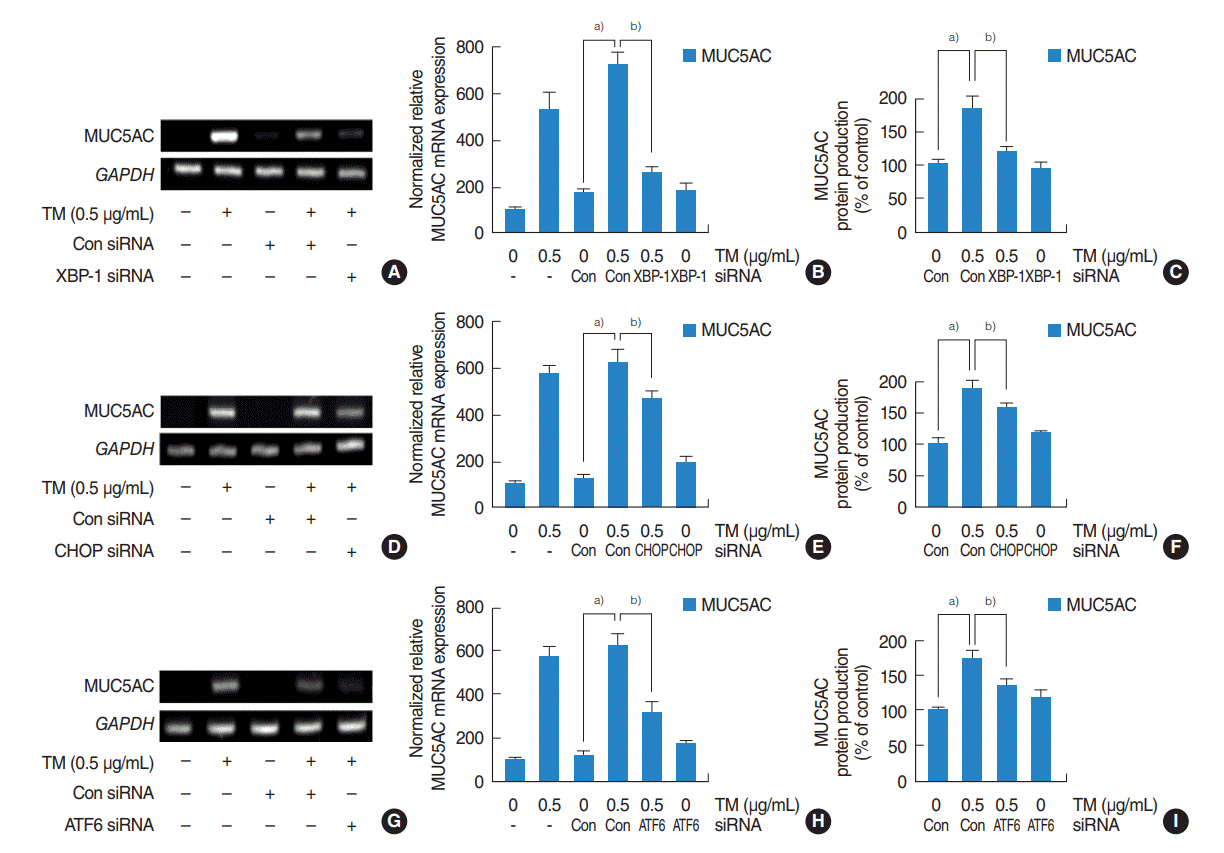 | Fig. 3.Contributions of the three major pathways to the upregulation of MUC5AC expression by endoplasmic reticulum stress in human nasal epithelial cells. (A-I) Reverse transcriptase-polymerase chain reaction (RT-PCR), real-time PCR and enzyme-linked immunosorbent assay showed that small interfering RNA (siRNA) knockdowns of XBP-1, CHOP, or ATF6 significantly blocked the endoplasmic reticulum stress-induced MUC5AC mRNA expression and glycoprotein production. (A-C) Inhibition by XBP-1 siRNA was greatest. (D-I) Inhibitions by CHOP and ATF6 siRNA were limited, and inhibition by CHOP siRNA was weakest. The images shown are representative of three separate experiments performed in triplicate. Bars represent the averages±standard deviation of three independent experiments performed in triplicate. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; TM, tunicamycin; XBP-1, X-box binding protein 1; Con, control; CHOP, CCAAT-enhancer-binding protein homologous protein; ATF6, activating transcription factor 6. a)P<0.05 vs. control siRNA. b)P<0.05 vs. TM (0.5 µg/mL) with control siRNA. |
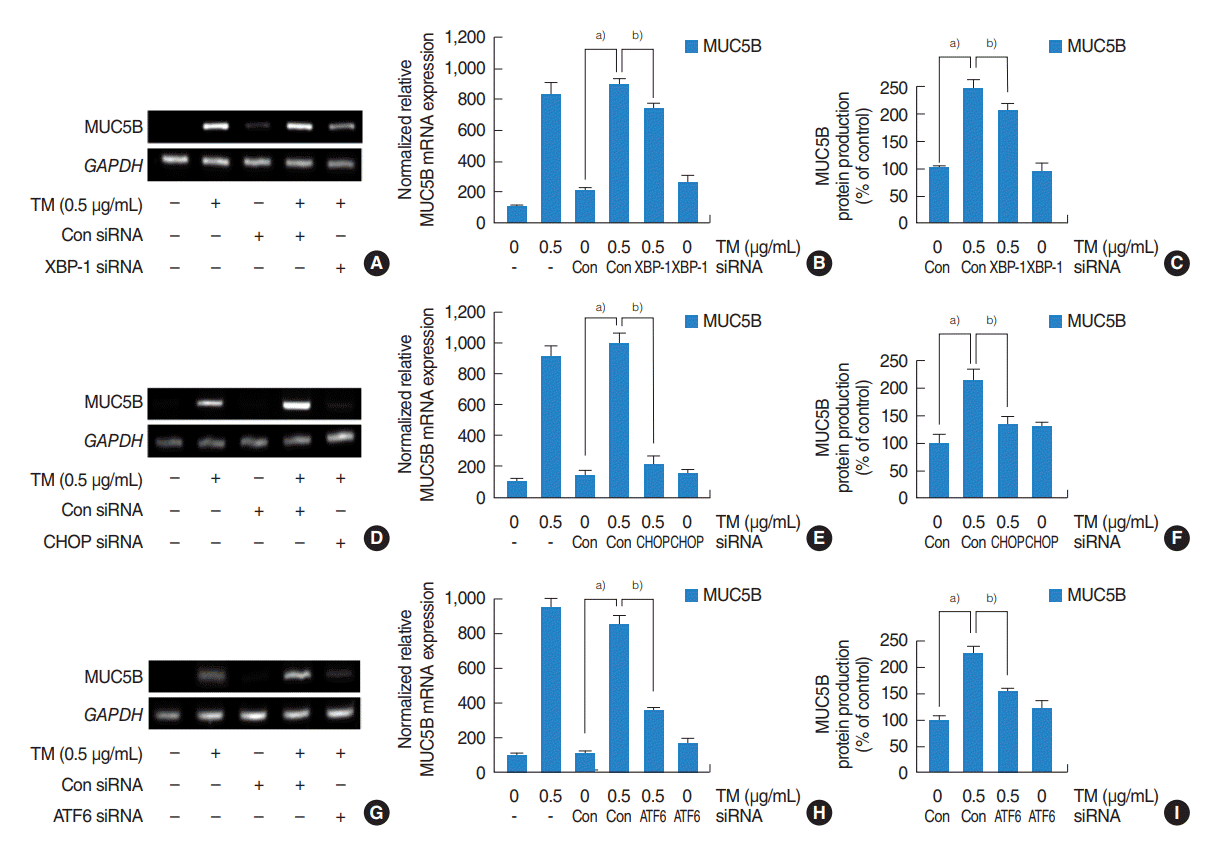 | Fig. 4.Contributions of the three major pathways to the upregulation of MUC5B expression by endoplasmic reticulum stress in human nasal epithelial cells. (A-I) Reverse transcriptase-polymerase chain reaction (RT-PCR), real-time PCR and enzyme-linked immunosorbent assay showed that small interfering RNA (siRNA) knockdowns of XBP-1, CHOP, or ATF6 significantly blocked the endoplasmic reticulum stress-induced MUC5B mRNA expression and glycoprotein production. (D-F) Inhibition by CHOP siRNA was greatest. (A-C, G-I) Inhibitions by XBP-1 and ATF6 siRNA were limited, and inhibition by XBP-1 siRNA was weakest. The images shown are representative of three separate experiments performed in triplicate. Bars represent the averages±standard deviation of three independent experiments performed in triplicate. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; TM, tunicamycin; Con, control; XBP-1, X-box binding protein 1; CHOP, CCAAT-enhancer-binding protein homologous protein; ATF6, activating transcription factor 6. a)P<0.05 vs. control siRNA. b)P<0.05 vs. TM (0.5 µg/mL) with control siRNA. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download