INTRODUCTION
Malignancy of the temporal bones is very rare [
1]. Metastases of primary malignancies to the temporal bones are known to be less common than temporal bone cancer. To date, the true incidence of temporal bone metastasis (TBM) is not yet well known [
2,
3]. However, the incidence seems to be gradually increasing because of increased cancer survival rates and the development of diagnostic techniques.
Various types of malignancies can metastasize to the temporal bone. It has been reported that not only solid cancers, such as lung cancer and breast cancer [
4], but hematologic malignancies, such as leukemia, also spread to the temporal bone [
5]. TBM occurs predominantly through hematologic spread, but may be caused by direct extension, meningeal carcinomatosis, or leukemic infiltration [
6]. To date, most studies on TBM have been case or postmortem studies on temporal bones [
7-
9]. Therefore, there are some difficulties in fully understanding the clinical features of TBM. It is also unclear whether there are any clinical differences between TBM from solid cancers and hematologic malignancies.
The purposes of this study were to analyze the clinical features of TBM and to determine whether there were any differences in the characteristics according to type of primary malignancy, which was divided into solid cancers and hematologic malignancies.
Go to :

MATERIALS AND METHODS
We retrospectively analyzed data on 20 patients diagnosed with TBM between January 2000 and January 2017 at the Department of Otolaryngology, Chungnam National University Hospital. Sixteen of the 20 patients were diagnosed based on histopathologic examination and the remaining four patients were diagnosed based on clinical symptoms, signs, and radiologic examination. Radiologically, TBM was defined when all three of the following imaging findings were satisfied: low-signal intensity in the T1-weighted image, low- or iso-signal intensity in the T2-weighted image, and contrast enhancement in the gadolinium-enhanced T1-weighted image. The study was approved by Institutional Review Board of Chungnam National University Hospital (IRB No. CNUH 2015-10-046) and informed consent was waived.
In each patient, the period from diagnosis of primary malignancy to diagnosis of TBM, the period from diagnosis of TBM to death, the type and staging of primary malignancy, symptoms and signs related to the ear, and sites of TBM were assessed. The metastatic sites to the temporal bone were classified as the temporal squama, petrous apex, internal auditory canal, external auditory canal, facial nerve, and middle ear cleft including the middle ear cavity and mastoid air cell. Cancer staging was classified according to the National Comprehensive Cancer Network guidelines for solid cancers and acute myeloid leukemia (AML). Cases of acute lymphocytic leukemia (ALL) were classified according to the presence of the Philadelphia chromosome. In addition, we compared these parameters after dividing the primary malignancies into solid cancers and hematologic malignancies to determine the differences between the two groups.
Statistical analysis was performed using the Mann-Whitney test for assessing the significance of differences in parametric data and Fisher exact test for analyzing nonparametric data. Differences were considered significant for probability values of P<0.05. All statistical analyses were performed using IBM SPSS ver. 22.0 (IBM Corp., Armonk, NY, USA).
Go to :

DISCUSSION
Various types of cancer are known to cause metastases to the temporal bones. In this study, there were patients with lung, kidney, breast, gastric, and bladder cancer and leukemia, such as AML and ALL, as primary malignancies with TBM. Among them, lung cancer was the most common primary cancer. This result is similar with a previous study that demonstrated that about 20% of TBM, including those in the internal auditory canal, resulted from lung cancer [
10]. However, other studies have shown that kidney cancer or breast cancer was the most common primary cancer with TBM [
11,
12]. Lung, kidney, and breast cancers must share common properties such that they have a predilection for disseminating to the bone [
13]. The differences in incidence may be due to the country or region in which the respective study was conducted. Lung cancer is a major primary cancer in countries with a large number of smokers, while breast cancer is a common primary malignancy in developed countries than in developing countries.
We noted that 16 out of the 18 patients in whom cancer staging could be determined were either at an advanced stage or at high risk. In addition, 12 out of 16 patients with solid cancers had other systemic metastases before TBM. Therefore, TBM seems to occur late in the disease process after the primary cancers first metastasize to other organs.
The most common clinical manifestations associated with TBM in the present study were facial palsy and hearing loss. These results are similar to those obtained in previous studies. Schuknecht et al. [
14] reported that 14.9% of patients with TBM showed facial palsy, which was slight lower than our incidence. Gloria-Cruz et al. [
11] reported that hearing loss was found in 40.4% of their subjects, which was the most common symptom in their study. Facial palsy and hearing loss are also common clinical manifestations of chronic otitis media. Therefore, if symptoms similar to chronic otitis media occur in patients with cancer, TBM of primary malignancy should be suspected.
In addition, none of our patients presented with the triad of symptoms (otalgia, periauricular swelling, and facial nerve paresis) as suggested by Maddox [
12], possibly because the symptom triad may have occurred when TBM had progressed considerably. Interestingly, only four patients had headache at the time of TBM diagnosis. Otologic symptoms such as facial palsy and hearing loss seem to be more important clues in TBM diagnosis than skull base symptoms such as headache.
We found that the temporal squama (23%) and the facial nerve (20%) were frequently involved in TBM. The internal auditory canal (18%) was also a common metastatic site. These metastatic sites coincided well with the clinical symptoms and signs. Previous studies have reported that the petrous apex was the most common metastatic site because not only is the petrous apex abundant in blood supply, but TBM also occurs mainly through hematogenous spread [
4,
11]. Interestingly, no metastasis to the inner ear was found in the current study. Gloria-Cruz et al. [
11] also reported that none of their 76 autopsy specimens had an involvement of the inner ear. This might be due to the poor vascularity of the inner ear including the otic capsule and membranous labyrinth compared to other parts of the temporal bone [
2].
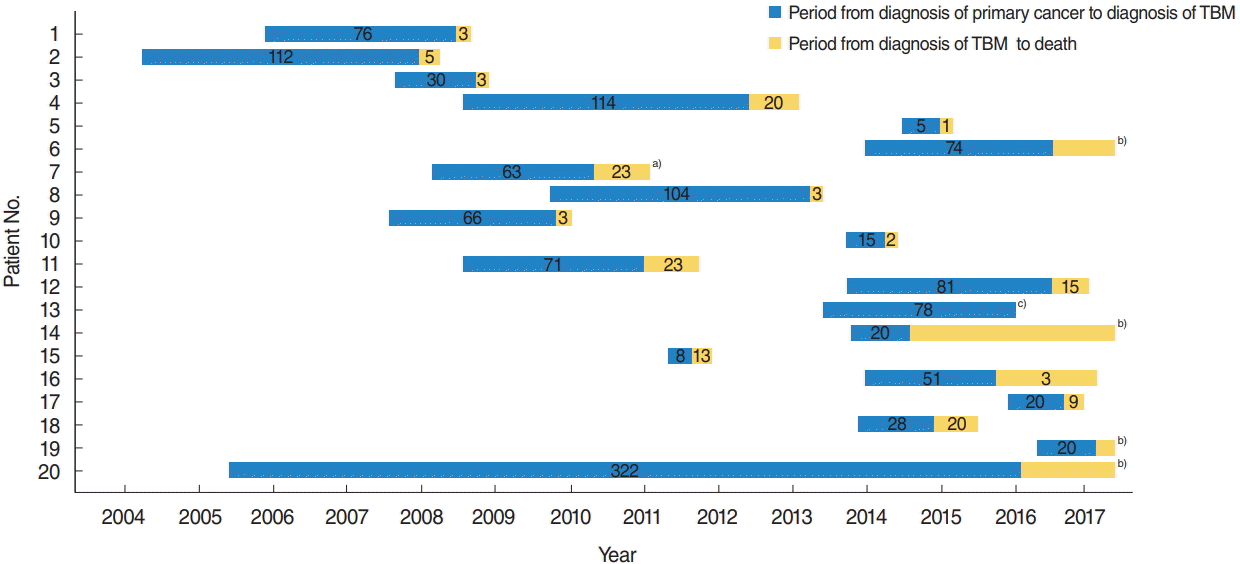
Although it has been previously reported that TBM to the internal auditory canal occurs many decades after primary cancer diagnosis [
15], we observed that the time from diagnosis of primary malignancy to diagnosis of TBM was an average of 22.5 months. The median time to death after diagnosis of TBM was 3.0 months, which was significantly shorter than the period from primary cancer diagnosis to diagnosis of TBM.
All patients received either chemotherapy or radiotherapy after TBM was diagnosed; however, all but four died. The majority of reports have also described poor prognosis with short survival times, similar to those shown in the present study [
8]. The prognosis could be attributed to poor general conditions and surgical difficulties in accessing sites of TBM.
We found that hematologic malignancies exhibit significantly more metastases to the external auditory canal and middle ear cleft than solid cancers do. Although the exact mechanism is unknown, this may be due to differences in metastatic patterns. Hematologic malignancies can spread to other organs by leukemic infiltration with submucous hemorrhage as well as hematogenous spread, whereas solid cancers mainly disseminate to distant sites via hematogenous pathways [
6,
11]. A histopathologic study showed that submucous hemorrhage and leukemic infiltration were major findings in temporal bones from patients with leukemia [
16]. In addition, infiltration and submucous hemorrhage in the external auditory canal are likely to be easier observed than those in other parts of the temporal bones because, anatomically, the external auditory canal is externally exposed.
This study has several limitations. First, the number of subjects was small. In particular, the number of patients with hematologic malignancies was small. Therefore, a further large-scale study is required to verify our findings. Second, the design of the study was retrospective. In addition, the sites of metastases were determined by imaging studies in areas where the biopsy could not be performed. Autopsy may have revealed different results. However, the majority of patients with TBM also have other systemic metastases, so they are in poor general condition and are unable to undergo biopsy or surgery. Some parts of the temporal bones, such as the petrous apex and the internal auditory canal, are also not easy to access. Therefore, the diagnosis of TBM in clinical settings is dependent on imaging tests. In the present study, four out of a total of 20 patients (20%) were diagnosed with TBM based on imaging studies, while the other 16 patients were confirmed to have metastases based on histologic examination.
In conclusion, the most common primary cancer that results in TBM is lung cancer. The metastases frequently present with facial palsy and hearing loss, which are similar with those of chronic otitis media. In addition, TBM is likely to occur when the primary cancer is advanced and has metastasized to other systems. Therefore, if otologic symptoms and signs, such as facial palsy or hearing loss, occur in patients with advanced primary cancer, we should have a high index of suspicion of TBM. Furthermore, hematologic malignancies tend to frequently disseminates to the external auditory canal and middle ear cleft compared to solid cancers.
Go to :





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download