Abstract
Objectives
As endoscopic instrumentation, techniques and knowledges have significantly improved recently, endoscopic ear surgery has become increasingly popular. Transcanal endoscopic ear surgery (TEES) can provide better visualization of hidden areas in the middle ear cavity during congenital cholesteatoma removal. We aimed to describe outcomes for TEES for congenital cholesteatoma in a pediatric population.
Methods
Twenty-five children (age, 17 months to 9 years) with congenital cholesteatoma confined to the middle ear underwent TEES by an experienced surgeon; 13 children had been classified as Potsic stage I, seven as stage II, and five as stage III. The mean follow-up period was 24 months. Recurrence of congenital cholesteatoma and surgical complication was observed.
Results
Congenital cholesteatoma can be removed successfully via transcanal endoscopic approach in all patients, and no surgical complications occurred; only one patient with a stage II cholesteatoma showed recurrence during the follow-up visit, and the patient underwent revision surgery. The other patients underwent one-stage operations and showed no cholesteatoma recurrence at their last visits. Two patients underwent second-stage ossicular reconstruction.
The classic presentation of congenital cholesteatoma (CC) is a whitish mass behind an intact tympanic membrane (TM) in young patients. Recently, the diagnosis of early-stage CC has increased because of growing interest in pediatric otitis media, the widespread use of diagnostic tools such as otoendoscopes and otomicroscopes in local clinics, and the prevalence of audiometric or tympanometric screening procedures [1]. The treatment of choice for CC is complete surgical removal, and early surgical intervention is advocated because CCs grow gradually in close relation to a patient’s age. Delays in detection and surgery may extend the disease [2] so that CCs subsequently spread to poorly visualized retrotympanic regions that can make them difficult to remove [3]. Additionally, the higher prevalence of otitis media and upper respiratory infections in children increase the factors that promote cholesteatoma growth [4]. When they are detected, CCs should be removed completely without causing surgical complications.
Postic’s staging system defines [5], four stages of CCs based on the occupied portion of the tympanum, presence of ossicular disruption, and mastoid extension, and the CC stage has been known to be strongly associated with residual and/or recurrent disease [5]. The proportions of residual and/or recurrent disease vary among studies [6-9] and depend mainly on stage, type (open or closed) and locations of CCs. Managing CCs in hidden areas, such as the sinus tympani, facial recess, anterior epitympanic space, and hypotympanum, in the middle ear is very important for reducing recurrent and/or residual CCs.
Advances in operative endoscopes have enabled transcanal endoscopic ear surgery (TEES) to treat middle ear disease [10-13], and the role of the endoscope has evolved in its application in managing cholesteatoma, particularly in the pediatric population [14-16]. Initially, endoscopes were used as adjuncts to microscopes and lower residual disease rates were reported when endoscopes were used at the end of cases to improve visualization of the tympanic cavity and for monitoring disease in second-look procedures [14]. Increasingly, endoscopes are utilized exclusively as the primary means of visualizing the operative field during surgical dissection, as a replacement for microscopes, with TEES possible when a mastoid is not necessary. The traditional operating microscope provides an excellent-quality magnified image in a straight line, but the surgeon’s field of view is limited to the narrowest segment of the ear canal; the endoscope allows a wider angle of view. In fact, depending on the angle of the endoscope, we can obtain wide fields of view in different directions, and therefore, the endoscope is greatly helpful in exploring the tympanic cavity in all directions and checking hidden areas supported by magnification. Therefore, TEES can provide better visualization of hidden areas in the middle ear cavity during CC removal.
Among CCs, stages I, II, and III are confined to the middle ear and can be removed by endoscopic approach alone. Recent studies have reported on the use of exclusively TEES for cholesteatoma removal [4,13,14,17,18]. However, data on patient outcomes following exclusive TEES are still lacking, especially for CCs in children. We present our experience with using TEES for CC removal and aim to determine the clinical efficacy of TEES during CC removal in a pediatric population.
We performed a retrospective review in 25 pediatric patients who underwent exclusive TEES for CC removal between June 2014 and March 2017 at tertiary referral hospitals. CC was defined as follows: (1) normal TM, (2) no continuity between the epithelium of the cholesteatoma and the TM, and (3) no history of otologic procedures. We did not consider history of otitis media with effusion [19].
The CCs had been classified as closed or open type based on operative findings [20], and Potsic stage [5] was determined as follows by the operative findings: stage I, disease confined to a single quadrant; stage II, cholesteatoma in multiple quadrants but without ossicular involvement or mastoid extension; stage III, ossicular involvement without mastoid extension; and stage IV, mastoid disease (not indicated for exclusive TEES).
All patients underwent high-resolution temporal bone computed tomography imaging before their operations, and one surgeon (IJM) performed all operations. Patients received routine follow-up at 3, 6, 12, 18, and 24 months postoperatively and annually thereafter at our center. Their TMs were observed by using an endoscope at each visit, and patients usually underwent a follow-up computed tomography (CT) scan 1 year after their operations. Pre- and/or postoperative play audiometry (15 patients) and pure tone audiometry (five patients, ≥4 or 5 years of age) were performed.
This study was approved by the Institutional Review Board of Samsung Medical Center (IRB No. 2017-09-017).
TEES was performed under general anesthesia; the surgeon primarily used one of two types of endoscopes with diameters of 3 mm, working lengths of 14 cm, and angles of either 0° or 30° (Karl Storz Endoscopy Korea, Seoul, Korea). The surgeon also used a 45° angled endoscope with a diameter of 3 mm to confirm complete removal of the cholesteatoma. The endoscopes were connected to a high-definition camera and a monitor to allow for the surgeon’s observation. After patients were injected with 2% lidocaine with 1:50,000 epinephrine, the tympanomeatal flap was elevated using a suction separator (2.5 mm) from a Panetti instrument set (Spiggle & Theis Medizintechnik, Overath, Germany). The tympanomeatal flap was elevated with 270° exposure. For instance, when the cholesteatoma was located in the anterior superior quadrant (ASQ) in the right ear, the surgeon made a circumferential incision with radial incisions at 10 and 4 o’clock in the skin of the external auditory canal. The TM was gently detached from the malleus except for the umbo. Thereafter, the cholesteatoma was removed using a curved dissector and hook; if the cholesteatoma matrix was too large to remove en bloc, the surgeon removed the matrix by dividing it into two or more pieces. In those cases, the surgeon opened the cholesteatoma sac and reduced its size by removing inner keratin debris. The surgeon carefully dissected the cholesteatomas from their surrounding structures while being careful to not lose hold of the sac. Great attention was paid to confirm that there was no residual cholesteatoma matrix after removal by exploring the tympanum with angled endoscopes. Posteriorly, the chorda was identified and preserved, and then the tympanomeatal flap was returned to its original position and covered with an absorbable gelatin sponge (Gelfoam; Pharmacia & Upjohn, New York, NY, USA).
Twenty-five children (age, 17 months to 9 years; average, 45 months) with CCs confined to the middle ear underwent TEES by an experienced surgeon between June 2014 and March 2017. The characteristics of all 25 patients are described in Table 1. There were 19 male and six female patients, and 16 right ears and nine left ears were affected.
The mean CC diameter on preoperative axial CT was 3.8 mm (range, 0.5 to 7.6 mm). Thirteen CCs were classified as Potsic stage I, seven as stage II, and five as stage III. The stage I cholesteatomas were mainly ASQ type (12 of 13), and stages II and III CCs were mainly of the post-quadrant type (10 of 12). Surgical findings demonstrated that 16 of the 25 ears had closed-type CCs and the other nine had open-type CCs (Table 1). The preoperative mean threshold of 20 patients who underwent play or pure tone audiometry was 17.7 dB HL (0.5, 1, 2, 4 kHz; range, 6.3 to 53.8 dB HL). Twenty-one of 25 patients underwent postoperative audiometry and their mean threshold was 18.9 dB HL (range, 6.3 to 43.8 dB HL). Of these 21, five patients with Potsic stage III CC that underwent tympanization had a postoperative mean threshold of 33.8 dB HL (range, 21.3 to 43.8 dB HL).
CC could be removed successfully via transcanal endoscopic approach in all patients, and no surgical complications in the middle or inner ear structures occurred. The mean postoperative follow-up period was 24±8.5 months (range, 12.2 to 37.3 months). Twenty of 25 patients underwent postoperative follow-up CT scans. Only one patient (case 9) who had a stage II CC showed residual or recurrent disease during a follow-up visit, and the patient underwent revision surgery via endoscopic approach 14 months after the first surgery. The recurred cholesteatoma was strongly attached to the medial side of the malleus handle, and it was completely removed with a small piece of the malleus handle; follow-up performed 16 months after the second operation revealed no evidence of recurrence. The other 24 patients who underwent TEES had developed no cholesteatoma recurrence at the last visit. Second stage ossicular reconstruction was performed in two patients, and their air-bone gaps improved successfully.
The following are representative patients of stage I CCs (ASQ-type) and stage III CCs (posterior inferior quadrant [PIQ]+posterior superior quadrant [PSQ]-type) treated by TEES. We present short case histories and details of the surgical procedures.
A 31-month-old female child was referred to our clinic for a whitish mass observed by an outside otolaryngologist. At the initial visit, a pearl-like round white mass was identified behind the ASQ of her right TM (Fig. 1A). Preoperative play audiometry revealed normal hearing (22.5 dB HL; 0.5, 1, 2, 4 kHz). CT demonstrated a 1.8-mm isolated, well-defined round mass of soft tissue density at the anterior aspect of the malleus handle (Fig. 1B). The patient was diagnosed with a right CC and underwent TESS under general anesthesia. The tympanomeatal flap was also elevated from the malleus short process and handle, leaving a portion attached to the umbo, and a closed-type round cholesteatoma was revealed at the ASQ in the tympanum (Fig. 1C); it was easily removed from surrounding structure with a dissector (Fig. 1D, E). The CC seemed to have originated from the cochleariform process, and the absence of cholesteatoma matrix within the tympanic cavity was confirmed after the CC was removed (Fig. 1F). The tympanomeatal flap was returned to its original position, and the postoperative course was uneventful. Follow-up at 11 months including CT evaluation revealed no evidence of recurrence.
A 4-year-old male visited an outside otolaryngologist because of suspicion of right hearing loss, and a white mass was found in the inferior half of the tympanic cavity through the intact TM; he was referred to our clinic with a suspicion of CC. At his initial visit, a relatively large white mass was recognized behind the PIQ and the anterior inferior quadrant (AIQ) of the intact TM (Fig. 2A), and pure tone audiometry revealed conductive hearing loss of 25 dB air-bone gap, bone conduction threshold 0 dB HL, and air conduction threshold 25 dB HL on pure tone average (0.5, 1, 2, 4 kHz) in the right ear (Fig. 2B). Preoperative CT scans demonstrated a mass of soft tissue density that occupied the mesotympanum and extended the sinus tympani with ossicular destruction (Fig. 2C, D), indicating CC. Posteriorly, the surgeon identified and preserved the chorda, and identified a closed-type CC that occupied the mesotympanum, mainly the posterior part of the tympanum, disrupting the long process of incus and tilting the stapes. The cholesteatoma was too large to remove en bloc, and thus the surgeon opened it and reduced its size by removing inner keratin debris. The surgeon carefully dissected the cholesteatoma from the surrounding structure while being careful not to lose hold of the sac and completely removed it including the cholesteatoma in the posterior tympanum (Fig. 3). After removing the CC, the surgeon explored the tympanum and confirmed whether there was residual cholesteatoma matrix in the tympanum using a 30° endoscope, returning the flap to its original position. A 10-month follow-up endoscopic exam (Fig. 4A) and CT scans showed no residual or recurrent cholesteatoma (Fig. 4B, C). One year after the first operation, the child underwent second stage ossiculoplasty with total ossicular replacement prosthesis, and the substantial air-bone gap decreased (Fig. 4D, E).
CC develops most frequently in the ASQ or PSQ of the tympanic cavity [20,21] and usually grows slowly and causes no symptoms until it finally results in anatomical and functional impairment such as ossicular destruction and hearing loss. Even in cases with hearing loss, children have difficulty complaining about their hearing loss because of their youth, and its diagnosis can be delayed more until the CC progresses to the whole tympanum or even the mastoid. Therefore, early surgical intervention is advocated because CC growth is closely linked to patient age, and delays in detection and treatment may facilitate extension of the disease [2]. Previously some authors recommended operative removal before the age of 3 years because CC detected thereafter is often accompanied by complications such as destruction of the ossicular chain [22]. Recently, easily available microscopic or endoscopic observation has resulted in easier diagnosis of CC [1]. The residual lesion rate in CC varies greatly among reports, but is consistent in terms of an increased residual ratio in advanced stages [20,23-25]. The residual ratio was high in patients in whom the CC was located medial to the malleus or the incus, with the CC abutting the incus or stapes, or the CC was enveloping or eroding the stapes [25]. Complete removal of cholesteatoma including hidden areas is essential in treating CC because cholesteatoma usually recurs when even a small piece of the cholesteatoma matrix remains in the tympanum. In this regard, endoscopes are very helpful for inspecting hidden areas at different angled views.
Conventional microscopic surgical views are often limited in visualizing the surroundings of CCs, especially they have adhered to the middle ear mucosa and posterior tympanum. Thus, only small CCs confined to well-visualized middle ear sub-sites could be removed by a transcanal approach using an operating microscope. For complete removal of more extensive CCs, more invasive procedures such as removing the ossicles and resecting the bony external auditory canal are often necessary in microscopic surgeries [26]. Recent advances in endoscopic technology have enabled removing cholesteatomas with rigid endoscopes through exclusive transcanal surgery in middle ear disease because endoscopes provide a wide-angle view [10,12,27,28]. Furthermore, angled endoscopes provide clear views of hidden areas such as the retrotympanum without the need for bone removal [29]. The most common site for ASQ-type CC is around the cochleariform process, and these can also be identified by endoscope.
Endoscopes were initially used as adjuncts to microscopes in cholesteatoma surgery to visualize poorly visualized regions, and several studies have reported lower rates of residual disease when using the endoscope combined with the microscope for rechecking and monitoring disease [14,30]. Thomassin et al. [30] initially reported the efficacy of endoscopic-assisted surgery and residual cholesteatoma in 47% of cases without endoscopes versus 6% in cases in which an endoscope was used. Additional systemic reviews have confirmed endoscopes identified residual disease in 16% to 76% of patients when the endoscope was used as a microscope adjunct during the primary procedure for observation and dissection [14]. The data suggest that adding an endoscope may reduce rates of residual or recurrent cholesteatoma in managing congenital or acquired cholesteatomas [12,30,31]. The role of the endoscope has evolved in its application in managing cholesteatoma, particularly in the pediatric population [14-16] because it can facilitate minimally invasive approaches. James et al. [15] reviewed 235 ears in 220 children who had had intact canal wall surgery; 108 underwent microscopic dissection with only endoscopic inspection, and 127 underwent increasing use of endoscopes for dissection. The authors found a 12% risk reduction in residual disease at 2.5 years when endoscopes were used for dissection, especially in the middle ear (22% vs. 11%). Cohen et al. [32] analyzed 25 patients who were undergoing second- or third-look procedures after cholesteatoma surgery, and 12 of these 25 procedures had been entirely endoscopic. Residual cholesteatoma was noted in nine of the 25 patients that were not detected on the primary microscopic dissection. Residual disease in the middle ear was located within the epitympanum sinus tympani or adjacent to the cochleariform process, where it might have been poorly visualized via microscope [32]. Patients who underwent TEES showed no significant difference in rate of tympanoplasty closure or, in mean hearing threshold improvement compared with those in non-TEES patients [32]. One recent study about TEES outcomes for pediatric cholesteatoma (congenital or acquired) similarly showed the lowest rates of recurrent and residual disease in patients who underwent TEES (recurrence rate of 12.9% and residual rate of 6.5%) [17]. They showed comparable operating times, recidivism rates, and audiological outcomes between cases that were endoscopically assisted and those that were primarily performed with an endoscope [17].
CC may have better outcomes because the pathology is not related to eustachian tube function [33]. CCs in Potsic stage I and II are good indicators of TEES, and a stage III CC involving ossicles can also be treated by TEES with an atticotomy. Few studies have reported outcomes of exclusively TEES for only CC in children, and this study was one of the relatively large number of studies to report CC outcomes with endoscopic approaches. A preliminary report by Kobayashi et al. [13] showed one case (stage III CC) of residual disease within 3 months of initial surgery in 12 patients with CC who underwent EES (8.3% residual rate). Ghadersohi et al. [17] recently reported on 65 pediatric congenital or acquired cholesteatoma cases including 11 CCs; in the CC cases, the endoscope was used as an adjunct to the microscope in one case, and TEES was performed in 10 cases. One of the 11 children revealed residual disease after surgery [17]. James et al. [15] reported cholesteatoma surgery outcomes including 29 CCs and compared residual rates between the group who underwent microscope surgery followed by endoscopic inspection and the group with endoscope-guided dissection. Analysis showed the endoscopic dissection was associated with less residua in the middle ear, but the authors did not differentiate the results for CC from those for acquired cholesteatoma.
The present study showed that 25 CCs localized in the middle ear could be safely treated with TEES without complications. Only one patient (4%) revealed a residual cholesteatoma and revision surgery was performed successfully via endoscopic approach; the other 24 patients exhibited no evidence of recurrence although our follow-up period was short. Two of five patients (a 4- and 5-year-old) underwent ossiculoplasty about 1 year after first TEES; the other three have not undergone ossiculoplasty yet. Opinions about performing one-stage surgery or planning second look operation with second stage ossiculoplasty can differ. We always confirm the absence of cholesteatoma recurrence after follow-up for at least 1 year. We certainly inform the parents about the need for second stage ossiculoplasty before and after surgery.
One consideration of TEES for CCs in children is the size of the external ear canal; in the transcanal approach, various surgical instruments need to be inserted through the external ear canal in addition to the endoscope. Our youngest patient was 17 months old, and the CC could be removed via entirely transcanal endoscopic approach with a 3.0-mm rigid endoscope without difficulty. In this study, the mean age was 45 months, and nearly all TEES cases were performed without difficulty or complications with the at least 3.0-mm rigid endoscope. Furthermore, in the majority of cases, there was no problem with using the 4-mm endoscope that is commonly used in nasal surgery. Young children generally have small external ear canals, which may make it difficult to perform TEES and require smaller diameter endoscope. The ability to perform TEES in small ear canals, however, depends on the site of the lesion (e.g., an ASQ lesion with anterior bony hanging in narrow ear canal).
In the pediatric population, the important advantages of TEES are improved visualization through a narrower ear canal, a broad dynamic view of the middle ear with the ability to “see around corners,” and a reduced rate of postauricular incision and dissection [12]. TEES is minimally invasive and bypasses the postauricular approach which is associated with potential surgical complications. The view through the microscope during the transcanal approach is firmly defined and limited by the narrowest portion of the ear canal. In contrast, the endoscope bypasses this narrow segment and provides a very wide view to the surgeon, even if a 0° endoscope is used. Inspections with a 30° or 45° enable access to the entire facial recess, anterior epitympanic space, entrance to the antrum, sinus tympani, and hypotympanum, which were previously inaccessible anatomical regions of the middle ear via the ear canal. Potential disadvantages of TEES is that it is a one-hand technique and surgeons who are used to a bimanual procedure may experience a learning curve to be fully familiar with them, in addition to the loss of binocular vision, with potentially longer initial surgical times and cost. Additionally, instruments designed for endoscopic procedures such as suction dissectors can sometimes be helpful.
Our study is a relatively large retrospective case series of TEES for pediatric CC cases, but there are inherent potential biases with any retrospective data series. All surgeries were performed by a single experienced surgeon who prefers the endoscopic approach and does not have a comparable series of non-TEES cases. The aim of this study was to show our experience with TEES for CC in children and present successful TEES outcomes that were comparable with the previously published literature, not to compare exclusively endoscopic versus conventional microscopic surgery for CC. It is certainly possible that there was a selection bias related to cholesteatoma stage, with entirely TEES not possible when mastoidectomy was required for extensive disease. Our cases comprised 13 stage I (52%), seven stage II (28%), and five stage III (20%) CCs. However, even in extensive cases, we found the endoscopes to be viable for approaching middle ear cholesteatoma.
Despite the short follow-up duration and small number of patients, the results from this suggest that CC can be safely removed using TEES and that comparable surgical outcomes can be achieved. The endoscopic approach is minimally invasive, makes no external scar, and provides better visualization of hidden areas in the middle ear. TEES has real advantages, especially in children, but surgeons do need experience to be fully familiar with one-hand surgery. Long-term follow-up results with more cases are needed.
▪ Twenty-five children with congenital cholesteatoma (CC) confined to the middle ear underwent transcanal endoscopic ear surgery (TEES).
▪ CC was removed successfully in all patients and only one patient showed recurrence.
▪ Pediatric CC limited to the middle ear cavity can be safely and effectively removed using TEES.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (Ministry of Science, ICT and Future Planning [MSIP], No. 2017R1 C1B5016610), Gwacheon, Republic of Korea.
REFERENCES
1. McGill TJ, Merchant S, Healy GB, Friedman EM. Congenital cholesteatoma of the middle ear in children: a clinical and histopathological report. Laryngoscope. 1991; Jun. 101(6 Pt 1):606–13.
2. Lim HW, Yoon TH, Kang WS. Congenital cholesteatoma: clinical features and growth patterns. Am J Otolaryngol. 2012; Sep-Oct. 33(5):538–42.

3. James AL. Endoscopic middle ear surgery in children. Otolaryngol Clin North Am. 2013; Apr. 46(2):233–44.

4. Marchioni D, Soloperto D, Rubini A, Villari D, Genovese E, Artioli F, et al. Endoscopic exclusive transcanal approach to the tympanic cavity cholesteatoma in pediatric patients: our experience. Int J Pediatr Otorhinolaryngol. 2015; Mar. 79(3):316–22.

5. Potsic WP, Samadi DS, Marsh RR, Wetmore RF. A staging system for congenital cholesteatoma. Arch Otolaryngol Head Neck Surg. 2002; Sep. 128(9):1009–12.

7. Doyle KJ, Luxford WM. Congenital aural cholesteatoma: results of surgery in 60 cases. Laryngoscope. 1995; Mar. 105(3 Pt 1):263–7.

8. Chen JM, Schloss MD, Manoukian JJ, Shapiro RS. Congenital cholesteatoma of the middle ear in children. J Otolaryngol. 1989; Feb. 18(1):44–8.
9. Sanna M, Zini C, Bacciu S. Surgery for cholesteatoma in children. In : Tos M, Thomsen J, Peitersen E, editors. Cholesteatoma and mastoid surgery. Amsterdam: Kugler and Ghedini Publication;1989. p. 685–8.
10. Migirov L, Shapira Y, Horowitz Z, Wolf M. Exclusive endoscopic ear surgery for acquired cholesteatoma: preliminary results. Otol Neurotol. 2011; Apr. 32(3):433–6.
11. Kakehata S, Futai K, Sasaki A, Shinkawa H. Endoscopic transtympanic tympanoplasty in the treatment of conductive hearing loss: early results. Otol Neurotol. 2006; Jan. 27(1):14–9.

12. Tarabichi M. Endoscopic management of acquired cholesteatoma. Am J Otol. 1997; Sep. 18(5):544–9.
13. Kobayashi T, Gyo K, Komori M, Hyodo M. Efficacy and safety of transcanal endoscopic ear surgery for congenital cholesteatomas: a preliminary report. Otol Neurotol. 2015; Dec. 36(10):1644–50.
14. Kozin ED, Gulati S, Kaplan AB, Lehmann AE, Remenschneider AK, Landegger LD, et al. Systematic review of outcomes following observational and operative endoscopic middle ear surgery. Laryngoscope. 2015; May. 125(5):1205–14.

15. James AL, Cushing S, Papsin BC. Residual cholesteatoma after endoscope-guided surgery in children. Otol Neurotol. 2016; Feb. 37(2):196–201.

16. Hunter JB, Zuniga MG, Sweeney AD, Bertrand NM, Wanna GB, Haynes DS, et al. Pediatric endoscopic cholesteatoma surgery. Otolaryngol Head Neck Surg. 2016; Jun. 154(6):1121–7.

17. Ghadersohi S, Carter JM, Hoff SR. Endoscopic transcanal approach to the middle ear for management of pediatric cholesteatoma. Laryngoscope. 2017; Nov. 127(11):2653–8.

18. Hanna BM, Kivekas I, Wu YH, Guo LJ, Lin H, Guidi J, et al. Minimally invasive functional approach for cholesteatoma surgery. Laryngoscope. 2014; Oct. 124(10):2386–92.

19. Levenson MJ, Parisier SC, Chute P, Wenig S, Juarbe C. A review of twenty congenital cholesteatomas of the middle ear in children. Otolaryngol Head Neck Surg. 1986; Jun. 94(5):560–7.

20. Kojima H, Tanaka Y, Shiwa M, Sakurai Y, Moriyama H. Congenital cholesteatoma clinical features and surgical results. Am J Otolaryngol. 2006; Sep-Oct. 27(5):299–305.

21. Hidaka H, Yamaguchi T, Miyazaki H, Nomura K, Kobayashi T. Congenital cholesteatoma is predominantly found in the posterior-superior quadrant in the Asian population: systematic review and meta-analysis, including our clinical experience. Otol Neurotol. 2013; Jun. 34(4):630–8.
22. Kim YH, Yoo JC, Lee JH, Oh SH, Chang SO, Koo JW, et al. Stage progression of congenital cholesteatoma in children. Eur Arch Otorhinolaryngol. 2012; Mar. 269(3):833–7.

23. Darrouzet V, Duclos JY, Portmann D, Bebear JP. Congenital middle ear cholesteatomas in children: our experience in 34 cases. Otolaryngol Head Neck Surg. 2002; Jan. 126(1):34–40.

24. Inokuchi G, Okuno T, Hata Y, Baba M, Sugiyama D. Congenital cholesteatoma: posterior lesions and the staging system. Ann Otol Rhinol Laryngol. 2010; Jul. 119(7):490–4.

25. Stapleton AL, Egloff AM, Yellon RF. Congenital cholesteatoma: predictors for residual disease and hearing outcomes. Arch Otolaryngol Head Neck Surg. 2012; Mar. 138(3):280–5.
26. Yamatodani T, Mizuta K, Hosokawa K, Takizawa Y, Sugiyama K, Nakanishi H, et al. Congenital middle ear cholesteatoma: experience from 26 surgical cases. Ann Otol Rhinol Laryngol. 2013; May. 122(5):316–21.

27. Marchioni D, Alicandri-Ciufelli M, Molteni G, Villari D, Monzani D, Presutti L. Ossicular chain preservation after exclusive endoscopic transcanal tympanoplasty: preliminary experience. Otol Neurotol. 2011; Jun. 32(4):626–31.
28. Tarabichi M. Endoscopic management of limited attic cholesteatoma. Laryngoscope. 2004; Jul. 114(7):1157–62.

29. Marchioni D, Alicandri-Ciufelli M, Piccinini A, Genovese E, Monzani D, Tarabichi M, et al. Surgical anatomy of transcanal endoscopic approach to the tympanic facial nerve. Laryngoscope. 2011; Jul. 121(7):1565–73.

30. Thomassin JM, Korchia D, Doris JM. Endoscopic-guided otosurgery in the prevention of residual cholesteatomas. Laryngoscope. 1993; Aug. 103(8):939–43.

31. Ito T, Kubota T, Watanabe T, Futai K, Furukawa T, Kakehata S. Transcanal endoscopic ear surgery for pediatric population with a narrow external auditory canal. Int J Pediatr Otorhinolaryngol. 2015; Dec. 79(12):2265–9.

Fig. 1.
Preoperative and operative findings in case 18. (A) Endoscopic findings revealed a white round mass behind the anterior superior quadrant of the tympanic membrane. (B) Computed tomography showed a well-defined round mass of soft tissue density at the anterior aspect of the malleus handle (arrow). A closed-type cholesteatoma (arrowhead) was recognized after a tympanomeatal flap was elevated (C) and removed completely (D, E). (F) The absence of congenital cholesteatoma was confirmed in the tympanic cavity including the cochleariform process (asterisk).
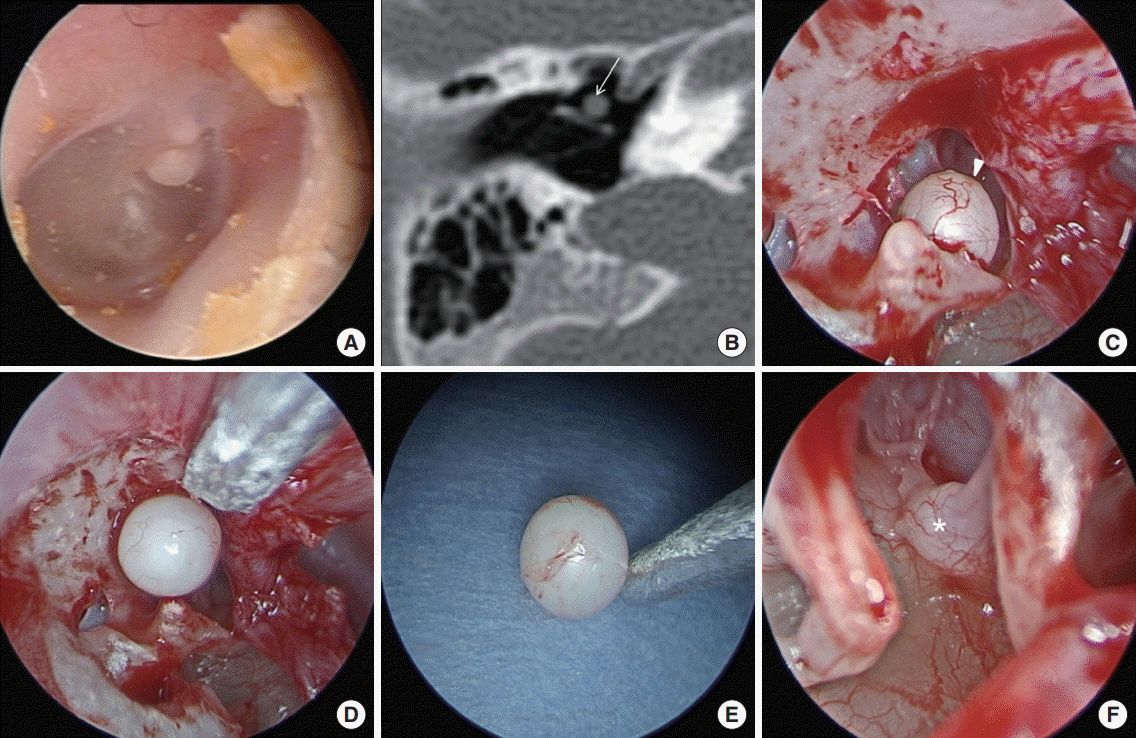
Fig. 2.
Preoperative findings in case 13. (A) Endoscopic findings revealed a white round mass behind the inferior half of the intact tympanic membrane. (B) Pure tone audiometry revealed conductive hearing loss of 25 dB air-bone gap in the right ear (red lines), while left hearing was normal (blue line). (C, D) Preoperative computed tomography scans demonstrated a mass of soft tissue density occupying the mesotympanum and extending sinus tympani with ossicular destruction.
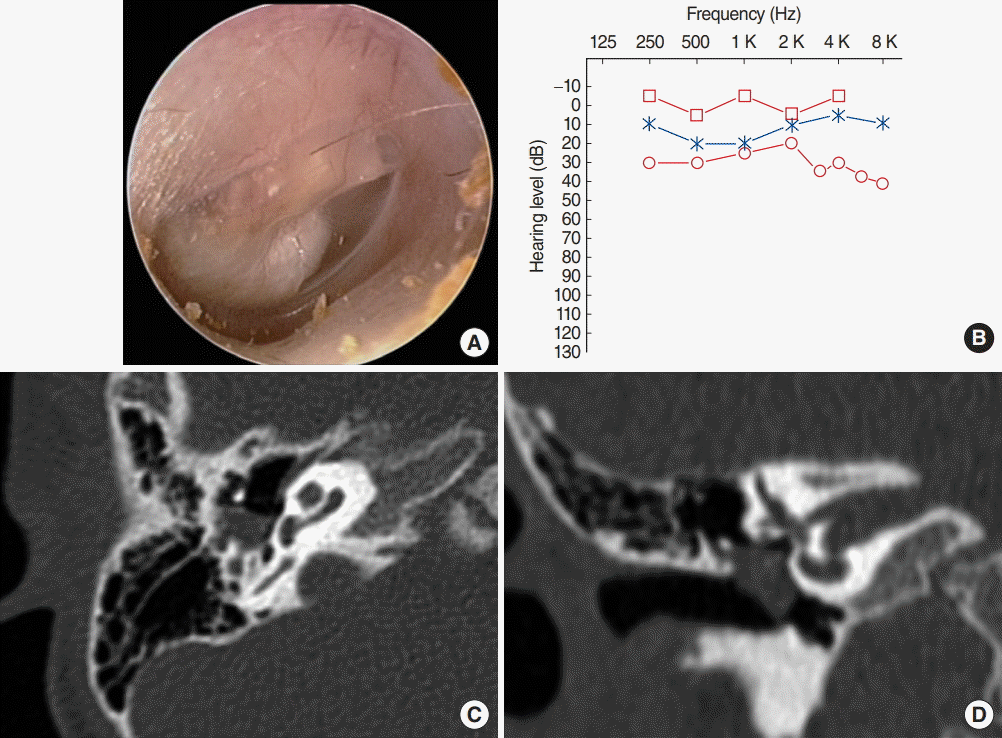
Fig. 3.
Operative findings in case 13. (A) Endoscopic findings revealed a large white mass occupying the tympanum behind the tympanomeatal flap. (B) The cholesteatoma was too large to remove en bloc, so it was opened and reduced in size by removing inner keratin debris. The cholesteatoma was carefully dissected from the surrounding structure while the surgeon was careful not to lose hold of the sac (arrow). The anterior part of the cholesteatoma was carefully dissected from the surrounding structure (C) and completely removed including in the posterior tympanum (arrows; D, E). (F) The absence of congenital cholesteatoma (CC) was confirmed in the tympanic cavity. CT, chorda tympani; Mh, malleus handle; S, stapes; RW, round window niche.
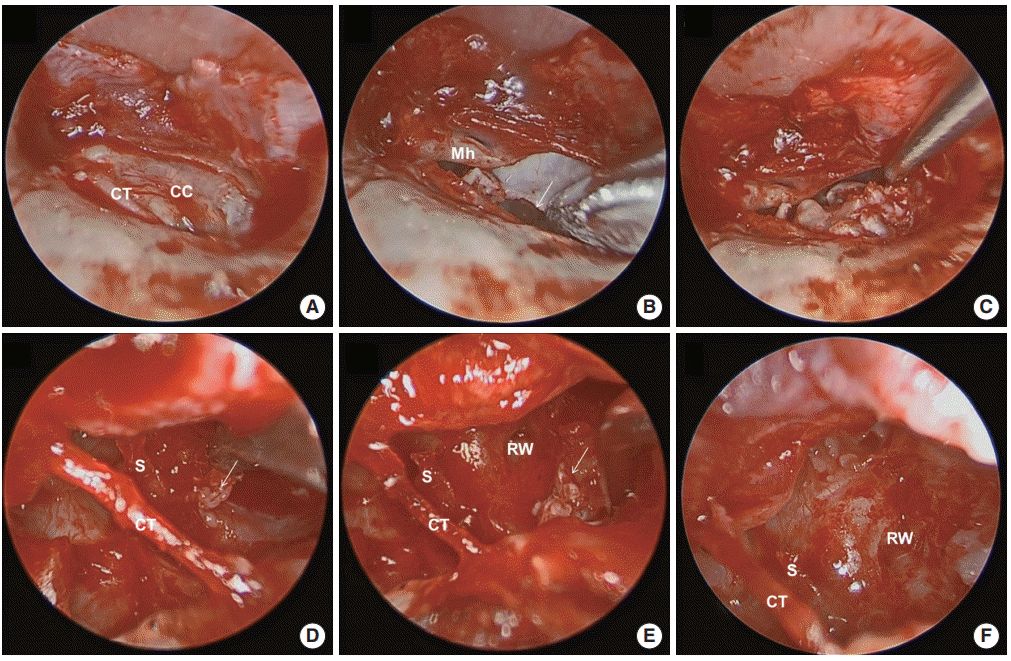
Fig. 4.
Postoperative findings in case 13. An endoscopic exam (A) and computed tomography scans (B, C, axial and coronal) showed no residual or recurrent cholesteatoma at 10-month follow-up. Air-bone gap in the right ear (red lines; left ear, blue line) (D) after the first operation, in which the destructed incus was removed with the congenital cholesteatoma, decreased substantially (E) after the second stage ossiculoplasty performed 1 year after congenital cholesteatoma removal.
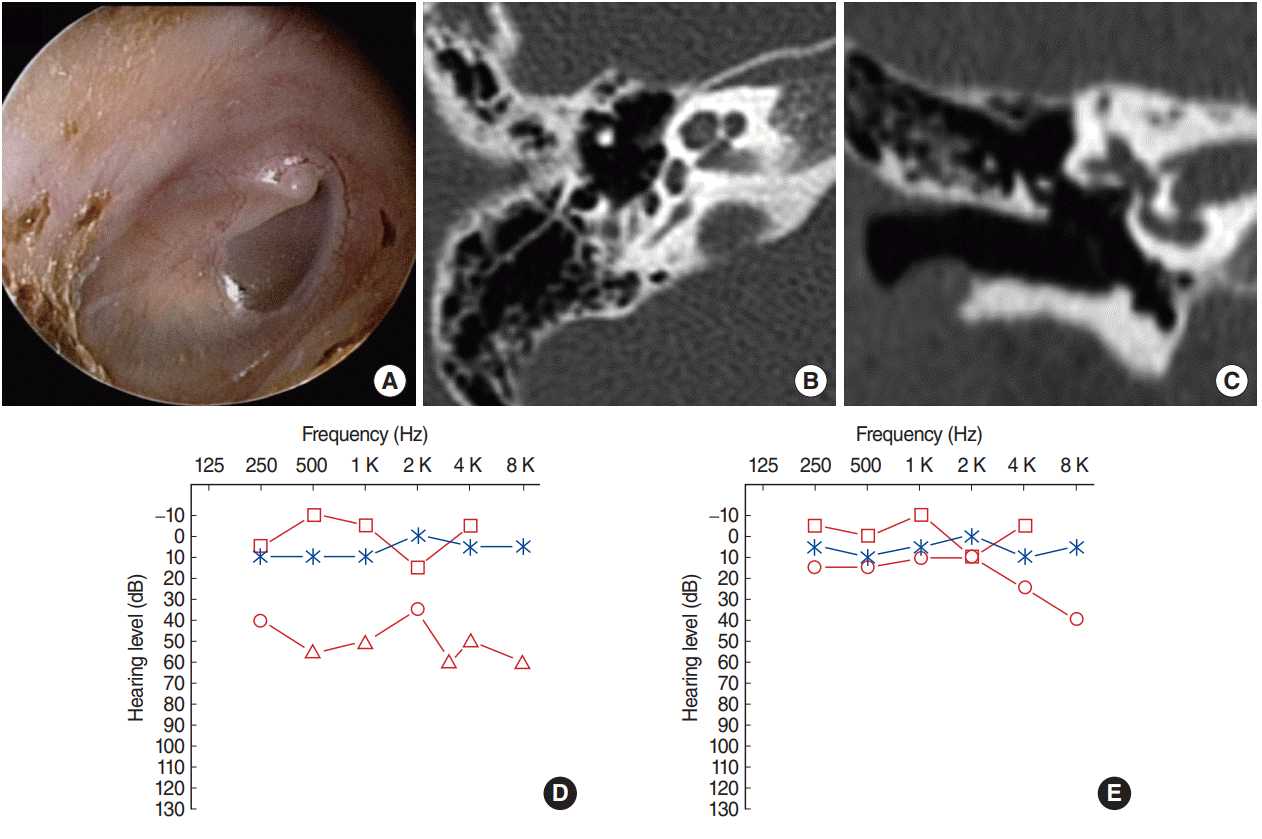
Table 1.
Demographics of patients and preoperative and operative findings
| No. | Sex | Age (mo) | Side | Diameter (mm)a) | Type | Site | Stage | Ossicle | Tympanoplasty | Recurrence | Revision |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 17 | Left | 0.5 | Closed | ASQ | I | Intact | Type I | – | |
| 2 | Male | 36 | Left | 6.0 | Closed | PSQ+PIQ | II | Intact | Type I | – | |
| 3 | Male | 36 | Right | 4.4 | Closed | PSQ+PIQ | II | Intact | Type I | – | |
| 4 | Male | 24 | Left | 6.8 | Open | PSQ+PIQ+ASQ | III | M, I, S anterior crura eroded | Tympanization | – | |
| 5 | Male | 60 | Left | 3.3 | Closed | ASQ | I | Intact | Type I | – | |
| 6 | Female | 48 | Left | 3.4 | Closed | PSQ+PIQ | II | Intact | Type I | – | |
| 7 | Male | 19 | Left | 1.1 | Closed | ASQ | I | Intact | Type I | – | |
| 8 | Male | 36 | Right | 4.1 | Open | ASQ | III | M, I eroded | Tympanization | – | |
| 9 | Male | 108 | Left | 3.9 | Open | ASQ+PSQ | II | Intact | Type I | + | Revision |
| 10 | Male | 33 | Right | 3.4 | Closed | PSQ+PIQ | II | Intact | Type I | – | |
| 11 | Male | 84 | Left | 7.6 | Open | PSQ+PIQ+ASQ | III | M, I, S suprastructure eroded | Tympanization | – | |
| 12 | Male | 36 | Right | 3.2 | Closed | ASQ | I | Intact | Type I | – | |
| 13 | Male | 48 | Right | 7.3 | Open | AIQ+PIQ | III | I long process eroded | Tympanization | – | Ossiculoplasty |
| 14 | Female | 60 | Right | 1.4 | Closed | PIQ | I | Intact | Type I | – | |
| 15 | Female | 24 | Right | 3.4 | Open | ASQ | I | Intact | Type I | – | |
| 16 | Male | 36 | Right | 3.7 | Closed | ASQ | I | Intact | Type I | – | |
| 17 | Female | 60 | Right | 3.5 | Open | PSQ | III | I, S suprastructure eroded | Tympanization | – | Ossiculoplasty |
| 18 | Female | 31 | Right | 1.8 | Closed | ASQ | I | Intact | Type I | – | |
| 19 | Male | 30 | Right | 3.8 | Closed | ASQ | I | Intact | Type I | – | |
| 20 | Male | 36 | Right | 2.2 | Closed | ASQ | I | Intact | Type I | – | |
| 21 | Male | 60 | Right | 4.1 | Open | ASQ+PSQ | II | Intact | Type I | – | |
| 22 | Male | 31 | Right | 4.7 | Open | ASQ | I | Intact | Type I | – | |
| 23 | Male | 36 | Right | 3.3 | Closed | ASQ | I | Intact | Type I | – | |
| 24 | Male | 23 | Left | 1.7 | Closed | ASQ | I | Intact | Type I | – | |
| 25 | Male | 30 | Right | 5.2 | Closed | ASQ+AIQ | II | Intact | Type I | – |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download