Abstract
Objectives
The current study aims to determine the correlation between nutritional status upon presentation and disease severity, as well as treatment and survival outcomes.
Methods
Patients who were diagnosed with deep neck infection, underwent at least one surgical drainage/debridement, and had more than 1 week of hospitalization at a tertiary medical center from 2007 to 2015 were retrospectively included. Thereafter, initial serum albumin, C-reactive protein (CRP), and body mass index (BMI) were reviewed.
Results
A total of 135 patients were included in the final analysis. Accordingly, the proportion of patients with simultaneous mediastinitis (21.0%), necrotizing fasciitis (12.9%), disease extent >1 cervical level (72.6%), mean CRP (22.4 mg/dL), mean length of hospitalization (25.0 days), and mean 1-week follow-up CRP (7.2 mg/dL) was significantly higher in the hypoalbuminemia group (initial serum albumin <3.0 g/dL) than in the normoalbuminemia group (all P<0.05). No significant correlations had been observed according to BMI status. After adjusting for age and Charlson comorbidity index, odds ratios for the following outcomes were calculated in patients initially presenting with hypoalbuminemia: simultaneous mediastinitis (3.07), necrotizing fasciitis (7.89), disease extent >1 cervical level (2.12), initial serum CRP over 20 mg/dL (3.79), hospitalization of more than 14 days (4.10), 1-week follow-up CRP over 5 mg/dL (3.78), and increased duration for an over 50% decrease in initial CRP (2.70) (all P<0.05). Although intravascular albumin replenishment decreased the proportion of patients with hypoalbuminemia after 2 weeks (P<0.05), it did not significantly predict better treatment outcomes.
Prompt assessment and selection of patients in need for surgical intervention should be the most crucial initial treatment strategy in the management of deep neck infections, as previously emphasized by many surgeons [1,2]. Infectious and inflammatory conditions of the human neck can cause rapid airway compromise [3]. Furthermore, the spread of infection through the prevertebral fascia could result in simultaneous mediastinitis or osteomyelitis of the cervical spine, which could be catastrophic for patient survival [4]. However, many physicians have argued that performing only physical examination of the neck is insufficient for the accurate assessment of severity and prediction of prognosis in patients with deep neck infection [5].
In 2004, Wong et al. [6] proposed a scoring system for predicting prognosis in necrotizing cervical fasciitis that included white blood cells, hemoglobin A1C, serum sodium, glucose, creatinine, and C-reactive protein (CRP). Their scoring system, termed Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC), was the first attempt to predict severe types of infections in the cervical area utilizing initial laboratory markers. However, the LRINEC scoring system showed weak predictability in cases where infections were exclusively limited to the deeper spaces of the neck [7,8].
In terms of critical care, low serum albumin levels were associated with poorer clinical outcomes and lower survival [9,10]. According to Vincent et al. [11], serum albumin level was an independent predictor of prognosis and treatment response in patients with acute distress. Additionally, in 2015, Moon [5] proposed a modified LRINEC scoring system that added initial serum albumin levels to the conventional LRINEC scoring system. Compared to the conventional scoring system, the modified LRINEC scores were shown to have higher modality in predicting clinical outcomes.
Serum albumin and body mass index (BMI) are widely accepted, easily measured indicators reflecting the overall nutritional status of an individual [12-14]. Therefore, the current study aimed to (1) compare the initial severity of deep neck infections between well-and poorly-nourished patients, (2) determine the risk for poorer clinical presentation and clinical outcomes, and (3) determine whether albumin replenishment could improve clinical outcomes.
Study subjects were recruited through retrospective medical chart review. Patients who primarily visited Asan Medical Center (a tertiary medical center) via the outpatient clinic or emergency room and were subsequently diagnosed with deep neck infection from January 2007 to December 2015 were reviewed. Patients with deep neck infection were defined as those who had undergone at least one surgical drainage/debridement of the infection source in addition to being hospitalized for more than 7 days. Patients under the age of 18 and those with deep cervical infection due to underlying malignancy, tuberculosis, trauma, and foreign material insertion to the head and neck area were excluded. Additionally, patients who had undergone contrastenhanced computed tomography (ceCT) or surgical debridement in other medical centers, as well as those referred from other medical centers, were excluded. This study was approved by the Institutional Review Board of Asan Medical Center (IRB No. 2017-0031).
All patients underwent ceCT of the head and neck area, as well as the chest area in cases where infectious spread along the mediastinum was evident. The diagnosis of deep neck infection in each patient was confirmed by an official reading from a boardcertified radiologist. Furthermore, two sub-categories of deep neck infections were established. Accordingly, patients whose ceCT showed an evident abscess pocket(s) in the deep cervical space were defined as the “deep cervical abscess” group, whereas those suffering from severe necrosis with soft-tissue infection spreading along the deep cervical fascia and cervical muscles were defined as the “necrotizing fasciitis” group. Abscess pockets exclusively located at the peritonsillar space, oropharyngeal/laryngeal mucosa, and salivary glands were not considered as true “deep neck infections” and therefore not included in the study.
Each patient had undergone surgical debridement followed by hospital admission. Decisions regarding patient treatment were made by a board-certified head and neck surgeon. Upon initial patient encounter, prompt resuscitation of vital signs, securing of the upper airway, and peripheral blood laboratory/microbiology tests had been performed. Intravenous (IV) antibiotics were immediately administered empirically based on established guidelines. Upon surgical drainage of deep cervical abscesses, a paired swab and tissue culture had been obtained for the identification of underlying microorganisms. Thereafter, the regimen and dosage of antibiotic agents were modified based on the results of the bacterial culture study and consultations with an infectious disease specialist. Cases where in the infection had spread to the mediastinum through the prevertebral space were consulted with a cardiothoracic surgeon, and surgical intervention and drainage of pus were performed as needed. Some patients with severe bacteremia and sepsis despite adequate surgical and medical treatment needed aggressive intervention, including critical patient care in intensive care unit admission. In cases with persisting hypoalbuminemia, single-to-multiple IV administrations of 100 mL of 20% albumin solution were considered. However, this could only be performed upon the informed consent from the patient or their guardians. Patients were discharged when they showed no evidence of active infection/inflammation and were tolerant to reasonable amounts of oral feeding.
Demographic information (age and sex) was reviewed. The severity of the underlying comorbidity in each patient was evaluated using the Charlson comorbidity index (CCI) in addition to reviewing the patients’ medical history of hypertension, diabetes, chronic liver disease, chronic pulmonary disease, end-stage/chronic renal disease, and coronary artery disease. The ceCT images were used to determine the extent of involvement (unilateral/bilateral), number of involved cervical levels, and presence of simultaneous mediastinitis. Peripheral blood laboratory results, including a complete blood count, chemical battery, and CRP, were available for all patients. Follow-up for the complete blood count, chemical battery, and CRP was done daily or every other day for the first and second week of treatment. For the remaining hospitalization period, blood tests were performed twice a week. Initial serum albumin and BMI were used to assess the nutritional state of each patient. Moreover, 1-week follow-up CRP and the duration for an over 50% decrease in the initial CRP were used to determine responses to treatment. Clinical outcomes were evaluated using the total number of surgical drainage, total length of hospitalization, and overall survival.
Values for or proportion of patients with each clinical factor according to the initial and 2-week nutritional status were compared using Student t-test, chi-square test, and Fisher exact test. Odds ratios (ORs) for the development of each clinical manifestation and worse clinical outcomes with poorer nutritional status, older age, and poorer underlying conditions were calculated using univariate logistic regression analysis. Multivariate analysis of the ORs for each clinical result was performed after adjusting for age and CCI. Survival curves were plotted using the Kaplan-Meier method, while the log-rank test was used to compare overall survival and elapsed time for a 50% reduction in serum CRP according to initial serum albumin levels. All of the tests were two-sided, and a P-value <0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS ver. 21.0 (IBM Corp., Armonk, NY, USA).
A total of 135 patients satisfied the inclusion criteria for the current study (Table 1), among which 50 (31.6%) and 85 (53.8%) accounted for males and females, respectively, with a median age of 62 years. Accordingly, 19 patients (14.1%) had hypertension, 46 (34.1%) diabetes, four (3.0%) chronic hepatitis, five (3.7%) end-stage renal disease (ESRD), two (1.5%) chronic pulmonary disease, and 19 (14.1%) coronary artery disease, resulting in 73 patients (54.1%) with a CCI ≥1. The median serum albumin level upon initial presentation was 3.1 g/dL with 62 patients (45.9%) having initial hypoalbuminemia (serum albumin <3.0 g/dL). Moreover, the median BMI value was 23.0 kg/m2 with 17 patients (12.6%) being underweight (BMI <18.5 kg/m2). A total of 30 patients (19.0%) showed bilateral disease involvement, while 85 (63.0%) had an infection that extended to more than one cervical level. The ceCT revealed a definite abscess pocket in 126 patients (93.3%) and necrotizing fasciitis in nine (6.7%). Simultaneous mediastinitis was observed in 19 patients (14.1%). The mean number of surgical drainage was 1.7 (standard deviation, 1.0) with a minimum of 1 and a maximum of 7, while the median duration of hospitalization was 14 days. Despite aggressive resuscitation and adequate control of the infection source, 11 patients (8.1%) died from sepsis followed by multiple organ failure.
Values for and proportion of patients with each clinical finding were compared according to initial serum albumin levels and BMI (Table 2). The proportion of patients with older age, simultaneous mediastinitis, necrotizing fasciitis, disease extent of more than one cervical level, and higher initial CRP was significantly greater in the hypoalbuminemia group than in the normoalbuminemia group (all P<0.05). The hypoalbuminemia group also showed poorer treatment responses and clinical outcomes, which resulted in longer duration of hospitalization, higher 1-week follow-up CRP, and longer time interval for a >50% decrease in the initial CRP level (all P<0.05). However, the number of surgical drainage and overall mortality rate did not differ between the two groups. On the other hand, no significant differences were found between the underweight and normalto-overweight BMI groups. ORs showed that individuals with initial serum albumin levels below 3.0 g/dL had a significant increase in the risk of developing simultaneous mediastinitis (2.96), necrotizing fasciitis (10.67), disease extent of more than one cervical level (2.18), and initial serum CRP over 20 mg/dL (4.20) (all P<0.05) (Table 3). In terms of clinical outcomes, the hypoalbuminemia group showed a significant increase in the risk for hospitalization of more than 14 days (4.90), 1-week followup CRP over 5 mg/dL (3.43), and longer time interval for an over 50% decrease in the initial CRP (3.10) (all P<0.05). Multivariate analysis showed significantly increased OR for more severe initial presentation and worse clinical outcomes as well (Table 4). To determine possible confounding clinical variables, univariate analysis was conducted for patients over 70 and those having a CCI >1 (Supplementary Table 1). In the elderly group (age >70 years), ORs suggested a significant increase in risk for necrotizing fasciitis (4.30), initial CRP >20 mg/dL (2.10), >14 days of hospitalization (3.11), and >1 week for a >50% decrease in the initial CRP (2.26). On the contrary, no significant increase/decrease in risk was observed for patients with CCI >1. Patients diagnosed with hepatic or renal failure showed a significant increase in the risk for necrotizing fasciitis (2.13), mediastinitis (2.16), and CRP >20 mg/dL (4.06) but not for clinical outcomes. Subsequently, a log-rank test was conducted for overall survival and time interval for a >50% decrease in initial CRP according to initial serum albumin levels, both of which showed worse outcomes and slower responses to treatment in the hypoalbuminemia group (both P<0.05) (Fig. 1).
A total of 73 patients hospitalized for longer than 14 days were assessed for their 2-week follow-up serum albumin levels and were subsequently categorized based on such findings. Hypoalbuminemia was observed in 41 patients (56.2%), whereas 32 (43.8%) had serum albumin levels greater than or equal to 3 g/dL (Supplementary Table 2). Despite the significantly higher proportion of patients receiving IV albumin replenishment in the normoalbuminemia group than in the hypoalbuminemia group (P<0.05), no significant differences in the demographic or clinical outcome parameters had been observed (Supplementary Table 2). In addition, IV albumin replenishment resulted in a 3.44-fold increase in the OR for 2-week normoalbuminemia and a 2.44-fold increase in the OR for less than 30 days of hospitalization (both P<0.05). However, ORs for overall survival and number of surgical drainage were not significant (Supplementary Table 3).
Based on results from 135 patients with deep neck infection, the present study showed that patients with initial hypoalbuminemia were likely to present with more severe clinical features and worse treatment outcomes. Although age over 70 years was another factor related to worse clinical manifestations, BMI and CCI were not. Patients who presented with serum albumin levels less than 3.0 g/dL were likely to have simultaneous mediastinitis, necrotizing fasciitis, greater extent of infection, higher initial CRP, delayed treatment response, and longer duration of hospitalization. Moreover, although IV albumin replenishment was able to decrease the proportion of patients with hypoalbuminemia after 2 weeks, it was not significant in the prediction of better treatment outcomes.
The anatomy of the deep cervical space is quite complicated given that it is surrounded by multiple layers of cervical fasciae [15]. Therefore, identifying infections in the deep cervical space remains challenging, especially during the early localized stage [16]. In addition, it is even harder to accurately assess the extent and severity of infections, which is essential in making prompt decisions regarding surgical intervention versus observation with IV antibiotics [17]. Given the risk for the spread of infection to the mediastinal cavity or vertebral space, many surgeons perform surgical drainage in most of the severe cases [18,19]. Boscolo-Rizzo et al. [20] had reported that among 365 patients with deep neck infections, 38.1% had to undergo surgical intervention, while 18.4% developed complications despite adequate therapy.
Therefore, many previous studies have focused on assessing the severity of deep neck infections through initial laboratory findings and radiologic results to determine the validity of surgical intervention [21-28]. In 2000, Wall et al. [17] were the first to report on the validity of serologic markers in diagnosing necrotizing fasciitis. Thereafter, Wong et al. [6] established a systemized scoring scale based on the results of the initial serologic study. However, the patients included in the aforementioned studies comprised a mixture of those diagnosed with deep neck infection, cellulitis, or relatively simple abscesses, e.g., peritonsillar abscesses, thereby limiting its application for patients with deep neck infections. Thomas and Meyer [29] had utilized multiple serum laboratory markers to determine the presence of deep cervical abscesses and necrotizing fasciitis in deep neck infection cases that had no potential for prediction of necrosis due to the small portion of necrotizing fasciitis among patients infected.
In 2012, Tsai et al. [30] had published an article showing that serum albumin levels were able to predict the prognosis of necrotizing fasciitis following surgical debridement, very similar to the results presented herein. Moon’s study [5], which included serum albumin in the LRINEC scoring system, showed increased scores for patients with necrotizing fasciitis which was significant. The present study showed a significantly high proportion of patients with necrotizing fasciitis in the low albumin group and that an initial serum albumin of <3.0 g/dL was an independent risk factor for an 8-fold increase in necrotizing fasciitis (P<0.05). These results are consistent with those from Moon’s study, thereby confirming that baseline nutritional state is somewhat related to the pathophysiology of disease. BMI, on the other hand, was not correlated with severity of disease or worse clinical outcomes.
In patients with chronic hepatitis or ESRD, where in protein loss or synthesis is taking place, we observed a 2- to 4-fold increase in the OR for necrotizing fasciitis, mediastinitis, and CRP above 20 mg/dL (all P<0.05) but not for clinical outcomes. Although the percentage of patients with decreased hepatic/renal function were considerably low (8%), the important role of albumin itself in potentially regulating the immunologic and inflammatory response becomes clearer, resulting in altered clinical outcomes when insufficient. Interestingly, 2-week serum albumin and IV albumin replenishment were not related to clinical outcomes. In similar fashion, administration of IV albumin had no benefit on survival and outcome in patients with septic shock. Together, these findings suggest that decreased serum albumin could be responsible for weakening the host’s immune system, thereby allowing rapid initial disease progression leading to a more severe and extensive state.
As proven in many previous studies, albumin plays a critical role in mediating inflammatory response, especially during the acute phase [9,11]. In response to systemic inflammation, the extravasation of albumin due to the increased vascular permeability leads to hypoalbuminemia in addition to the rapid breakdown of amino acids, further contributing to a decline in serum albumin levels [31]. When administered intravascularly, albumin has its own function in terms of regulating enzymatic activity, acidity, and electrolyte balance, thereby maintaining homeostasis in our body [10]. Hypoalbuminemia is thought to be both a cause and result of systemic inflammation, resulting in worsening and progression of the inflammatory reaction [32]. On the other hand, albumin is an important molecule in maintaining the osmotic pressure in the intravascular space [11]. A decline in the intravascular albumin can lead to edematous conditions in many organs, resulting in severe complications other than the infection itself, such as pneumonia or pleural effusion [33]. Together, these findings support the ability of initial serum albumin to predict prognosis and initial disease extent. Although a randomized control trial had been conducted on aggressive parenteral nutritional support during the acute phase of inflammation, its results did not show improvement in outcomes [34]. Therefore, we believe that initial serum albumin is a valid tool for assessing disease severity and predicting clinical outcomes; however, manipulation of serum albumin levels does not bring promising treatment results.
Although our study confirmed the clinical validity of initial serum albumin levels in predicting more severe disease and poorer clinical outcomes, we do acknowledge the following study limitations: (1) due to the retrospective study design, we were not able to obtain other laboratory and physical markers that represent an individual’s nutritional status; (2) IV albumin replenishment had not been uniformly administered in terms of dosage, duration, and formula; (3) the lack of a previously established nutritional parameter (e.g., Malnutrition Universal Screening Tool or Nutritional Risk Screening 2002 scoring system), which could have allowed for a more comprehensive evaluation of nutritional status compared to the use of only serum albumin levels and BMI [35-37]. Therefore, we suggest the need for future prospective studies that accurately assess initial nutritional status, including a full-nutrition battery (e.g., folate, selenium, cobalamin, and arm circumference measurement), and utilize established clinical protocols for IV albumin replenishment.
Initial serum albumin levels in patients presenting with deep neck infection was an independent serologic marker predicting more severe and complicated disease states. Additionally, initial serum albumin less than 3.0 g/dL was an independent factor predicting worse clinical outcomes, such as longer hospitalizations and slower responses to treatment. Although IV albumin replenishment was able to correct hypoalbuminemia, it did not lead to better clinical outcomes. Our results indicate that patients with deep neck infections who present with hypoalbuminemia should be evaluated and managed more cautiously.
▪ Risk factors of complicated deep neck infections were retrospectively examined in 135 patients.
▪ Initial serum albumin less than 3.0 g/dL was an independent serologic marker to predict complicated deep neck infection (P<0.05).
▪ Intravenous albumin replenishment did not show a significance in the prediction of better treatment outcomes.
ACKNOWLEDGMENTS
The authors would like to show a gratitude to all medical staffs who participated in the treatment of the patients included for the study by offering their best professional care.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.21053/ceo.2018.00108.
Supplementary Table 1.
Univariate analyses of patient age and general condition with initial clinical presentation, treatment response, and clinical
outcome (n=135)
Supplementary Table 2.
Differences in the clinical manifestations according to 2-week serum albumin level (n=73)
Supplementary Table 3.
Univariate and multivariate analyses of the clinical outcome in patients who did not receive any intravenous albumin
replenishment (n=73)
REFERENCES
1. Brito TP, Hazboun IM, Fernandes FL, Bento LR, Zappelini CE, Chone CT, et al. Deep neck abscesses: study of 101 cases. Braz J Otorhinolaryngol. 2017; May-Jun. 83(3):341–8.

2. Vieira F, Allen SM, Stocks RM, Thompson JW. Deep neck infection. Otolaryngol Clin North Am. 2008; Jun. 41(3):459–83.

3. Kataria G, Saxena A, Bhagat S, Singh B, Kaur M, Kaur G. Deep neck space infections: a study of 76 cases. Iran J Otorhinolaryngol. 2015; Jul. 27(81):293–9.
4. Mazzella A, Santagata M, Cecere A, La Mart E, Fiorelli A, Tartaro G, et al. Descending necrotizing mediastinitis in the elderly patients. Open Med (Wars). 2016; Nov. 11(1):449–60.

5. Moon JH. Usefulness of the modified LRINEC score in the treatment of patient with deep neck infection. Korean J Otorhinolaryngol Head Neck Surg. 2015; Feb. 58(2):115–9.

6. Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004; Jul. 32(7):1535–41.

7. Wong CH, Khin LW. Clinical relevance of the LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score for assessment of early necrotizing fasciitis. Crit Care Med. 2005; Jul. 33(7):1677.

8. Wong CH, Wang YS. The diagnosis of necrotizing fasciitis. Curr Opin Infect Dis. 2005; Apr. 18(2):101–6.

9. Casaer MP, Van den Berghe G. Nutrition in the acute phase of critical illness. N Engl J Med. 2014; Mar. 370(13):1227–36.

10. Shaw JH, Koea JB. Metabolic basis for management of the septic surgical patient. World J Surg. 1993; Mar-Apr. 17(2):154–64.

11. Vincent JL, Dubois MJ, Navickis RJ, Wilkes MM. Hypoalbuminemia in acute illness: is there a rationale for intervention? A meta-analysis of cohort studies and controlled trials. Ann Surg. 2003; Mar. 237(3):319–34.
12. Blackburn GL, Bistrian BR, Maini BS, Schlamm HT, Smith MF. Nutritional and metabolic assessment of the hospitalized patient. JPEN J Parenter Enteral Nutr. 1977; 1(1):11–22.

13. Keys A, Fidanza F, Karvonen MJ, Kimura N, Taylor HL. Indices of relative weight and obesity. Int J Epidemiol. 2014; Jun. 43(3):655–65.

14. Morey VM, Song YD, Whang JS, Kang YG, Kim TK. Can serum albumin level and total lymphocyte count be surrogates for malnutrition to predict wound complications after total knee arthroplasty. J Arthroplasty. 2016; Jun. 31(6):1317–21.

15. Hirasawa K, Tsukahara K, Motohashi R, Endo M, Sato H, Ueda Y, et al. Deep neck cellulitis: limitations of conservative treatment with antibiotics. Acta Otolaryngol. 2017; Jan. 137(1):86–9.

16. Olejniczak I, Bojanowska-Pozniak K, Pietruszewska W. Deep neck infections: still important diagnostic and therapeutic problem. Otolaryngol Pol. 2016; Apr. 70(2):25–30.
17. Wall DB, Klein SR, Black S, de Virgilio C. A simple model to help distinguish necrotizing fasciitis from nonnecrotizing soft tissue infection. J Am Coll Surg. 2000; Sep. 191(3):227–31.
18. Celakovsky P, Kalfert D, Tucek L, Mejzlik J, Kotulek M, Vrbacky A, et al. Deep neck infections: risk factors for mediastinal extension. Eur Arch Otorhinolaryngol. 2014; Jun. 271(6):1679–83.

19. Gausepohl JS, Wagner JG. Survival from cervical necrotizing fasciitis. West J Emerg Med. 2015; Jan. 16(1):172–4.

20. Boscolo-Rizzo P, Stellin M, Muzzi E, Mantovani M, Fuson R, Lupato V, et al. Deep neck infections: a study of 365 cases highlighting recommendations for management and treatment. Eur Arch Otorhinolaryngol. 2012; Apr. 269(4):1241–9.

21. Staffieri C, Fasanaro E, Favaretto N, La Torre FB, Sanguin S, Giacomelli L, et al. Multivariate approach to investigating prognostic factors in deep neck infections. Eur Arch Otorhinolaryngol. 2014; Jul. 271(7):2061–7.

22. Obregon-Guerrero G, Martinez-Ordaz JL, Moreno-Aguilera E, Ramirez-Martinez M, Pena-Garcia JF, Perez-Alvarez C. Deep neck abscess: factors related to reoperation and mortality. Cir Cir. 2013; Jul-Aug. 81(4):299–306.
23. Sandner A, Moritz S, Unverzagt S, Plontke SK, Metz D. Cervical necrotizing fasciitis: the value of the laboratory risk indicator for necrotizing fasciitis score as an indicative parameter. J Oral Maxillofac Surg. 2015; Dec. 73(12):2319–33.
24. Barber BR, Dziegielewski PT, Biron VL, Ma A, Seikaly H. Factors associated with severe deep neck space infections: targeting multiple fronts. J Otolaryngol Head Neck Surg. 2014; Aug. 43(1):35.

25. Cardenas-Malta KR, Cortes-Flores AO, Fuentes-Orozco C, Martinez-Oropeza Ldel C, Lopez-Ramirez MK, Gonzalez-Ojeda A. Necrotizing mediastinitis in deep neck infections. Cir Cir. 2005; Jul-Aug. 73(4):263–7.
26. Cheng Z, Yu J, Xiao L, Lian Z, Wei Y, Wang J. Deep neck infection: clinical analyses of 95 cases. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2015; Sep. 50(9):769–72.
27. Huang TT, Tseng FY, Yeh TH, Hsu CJ, Chen YS. Factors affecting the bacteriology of deep neck infection: a retrospective study of 128 patients. Acta Otolaryngol. 2006; Apr. 126(4):396–401.

28. Yang W, Hu L, Wang Z, Nie G, Li X, Lin D, et al. Deep neck infection: a review of 130 cases in southern China. Medicine (Baltimore). 2015; Jul. 94(27):e994.
29. Thomas AJ, Meyer TK. Retrospective evaluation of laboratory-based diagnostic tools for cervical necrotizing fasciitis. Laryngoscope. 2012; Dec. 122(12):2683–7.

30. Tsai YH, Huang KC, Shen SH, Hsu WH, Peng KT, Huang TJ. Microbiology and surgical indicators of necrotizing fasciitis in a tertiary hospital of southwest Taiwan. Int J Infect Dis. 2012; Mar. 16(3):e159–65.

31. Allingstrup MJ, Esmailzadeh N, Wilkens Knudsen A, Espersen K, Hartvig Jensen T, Wiis J, et al. Provision of protein and energy in relation to measured requirements in intensive care patients. Clin Nutr. 2012; Aug. 31(4):462–8.

32. Plank LD, Connolly AB, Hill GL. Sequential changes in the metabolic response in severely septic patients during the first 23 days after the onset of peritonitis. Ann Surg. 1998; Aug. 228(2):146–58.

33. Geng Y, Qi Q, Sun M, Chen H, Wang P, Chen Z. Prognostic nutritional index predicts survival and correlates with systemic inflammatory response in advanced pancreatic cancer. Eur J Surg Oncol. 2015; Nov. 41(11):1508–14.

34. Casaer MP, Wilmer A, Hermans G, Wouters PJ, Mesotten D, Van den Berghe G. Role of disease and macronutrient dose in the randomized controlled EPaNIC trial: a post hoc analysis. Am J Respir Crit Care Med. 2013; Feb. 187(3):247–55.
35. Jones JM. The methodology of nutritional screening and assessment tools. J Hum Nutr Diet. 2002; Feb. 15(1):59–71.

Fig. 1.
Overall survival and response to treatment according to the initial serum albumin level. Kaplan-Meier curves estimating the overall survival (A) and more than 50% reduction in initial serum C-reactive protein (CRP) (B), suggesting lower treatment responses and worse clinical outcomes in patients with initial hypoalbuminemia (P<0.05, both). Log-rank test, P<0.05.
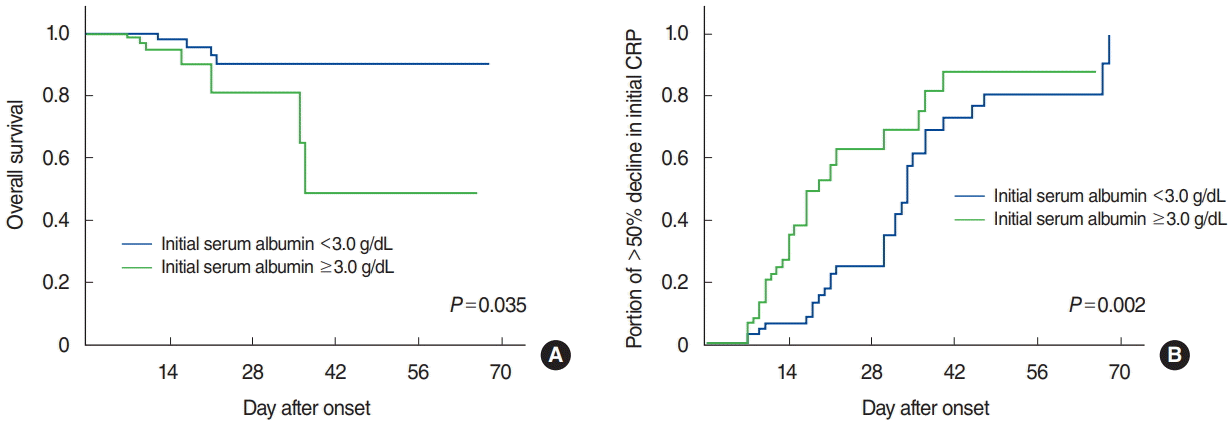
Table 1.
Patient characteristics
Table 2.
Differences in clinical manifestations according to nutritional status
| Patient demographics | Hypoalbuminemia (serum albumin <3.0 g/dL, n=62) | Normoalbuminemia (serum albumin ≥3.0 g/dL, n=73) | P-valuea) | Underweight (BMI <18.5 kg/m2, n=17) | Normal-to-overweight (BMI ≥18.5 kg/m2, n=118) | P-valuea) |
|---|---|---|---|---|---|---|
| Age (yr) | 67.8±13.3 | 56.1±15.8 | <0.001b) | 59.6±20.2 | 61.7±15.1 | 0.612 |
| CCI >1 | 37 (59.7) | 36 (49.3) | 0.229 | 8 (47.1) | 65 (55.1) | 0.535 |
| Clinical & laboratory findings on initial presentation | ||||||
| Simultaneous mediastinitis | 13 (21.0) | 6 (8.2) | 0.034b) | 4 (23.5) | 15 (12.7) | 0.261 |
| Necrotizing fasciitis | 8 (12.9) | 1 (1.4) | 0.012b) | 0 | 9 (7.6) | 0.602 |
| Bilateral involvement | 17 (27.4) | 13 (17.8) | 0.181 | 4 (23.5) | 26 (22.0) | 1.000 |
| Extended to >1 cervical level | 45 (72.6) | 40 (54.8) | 0.033b) | 10 (58.8) | 75 (63.6) | 0.705 |
| WBC (×106/L) | 10,600.3±7,550.6 | 9,164.5±6,872.5 | 0.445 | 12,727.2±4,784.0 | 11,943.8±4,402.0 | 0.499 |
| CRP (mg/dL) | 22.4±9.8 | 17.4±8.8 | 0.002b) | 18.5±11.4 | 19.9±9.3 | 0.578 |
| Clinical outcome and response to the treatment | ||||||
| Length of hospitalization (day) | 25.0±14.6 | 14.6±10.9 | <0.001b) | 21.1±18.7 | 19.1±12.1 | 0.584 |
| No. of surgical drainage | 1.8±1.2 | 1.6±0.8 | 0.248 | 1.8±0.9 | 1.7±1.0 | 0.716 |
| No. of dead patients | 4 (6.5) | 7 (9.6) | 0.548 | 1 (5.9) | 10 (8.5) | 1.000 |
| 1-Week follow-up CRP (mg/dL) | 7.2±5.2 | 4.4±4.6 | 0.001b) | 6.3±6.2 | 5.6±4.9 | 0.547 |
| Time interval for 50% decrease of initial CRP (day) | 6.4±4.2 | 5.0±3.0 | 0.029b) | 6.1±3.9 | 5.5±3.6 | 0.669b) |
Values are presented as mean±standard deviation or number (%).
BMI, body mass index; CCI, Charlson comorbidity index; WBC, white blood cell; CRP, C-reactive protein.
Table 3.
Univariate analyses of nutritional markers according to initial clinical presentation, treatment response, and clinical outcomes
| Variable |
Hypoalbuminemia (serum albumin <3.0 g/dL) |
Underweight (BMI <18.5 kg/m2) |
||
|---|---|---|---|---|
| OR (95% CI)a) | P-value | OR (95% CI)a) | P-value | |
| Initial presentation | ||||
| Simultaneous mediastinitis | 2.96 (1.05–8.34) | 0.040b) | 2.11 (0.61–7.34) | 0.239 |
| Necrotizing fasciitis | 10.67 (1.30–87.86) | 0.028b) | 1.29 (0.10–1.34) | 0.989 |
| Bilateral involvement | 1.74 (0.77–3.96) | 0.183 | 1.09 (0.33–3.62) | 0.890 |
| Extended to >1 cervical level | 2.18 (1.06–4.50) | 0.034b) | 0.82 (0.29–2.31) | 0.706 |
| Serum CRP >20 mg/dL | 4.20 (2.04–8.65) | <0.001b) | 1.13 (0.41–3.13) | 0.817 |
| Clinical outcomes and treatment response | ||||
| Length of hospitalization >14 days | 4.90 (2.33–10.28) | <0.001b) | 0.95 (0.34–2.63) | 0.920 |
| No. of surgical drainage >1 | 1.26 (0.64–2.49) | 0.508 | 1.53 (0.55–4.24) | 0.414 |
| Death | 1.65 (0.18–4.33) | 0.509 | 0.68 (0.08–5.63) | 0.717 |
| 1-Week follow-up CRP >5 mg/dL | 3.43 (1.68–7.01) | 0.001b) | 1.25 (0.45–3.47) | 0.666 |
| Time interval for 50% decrease of initial CRP >7 days | 3.10 (1.36–7.08) | 0.007b) | 1.84 (0.62–5.43) | 0.271 |
Table 4.
Multivariate analysesa) of hypoalbuminemia according to initial clinical presentation, treatment response, and clinical outcome
| Variable |
Hypoalbuminemia (serum albumin <3.0 g/dL) |
|
|---|---|---|
| OR (95% CI)b) | P-value | |
| Initial presentation | ||
| Simultaneous mediastinitis | 3.07 (1.04–9.08) | 0.043c) |
| Necrotizing fasciitis | 7.89 (0.91–68.05) | 0.060c) |
| Bilateral involvement | 1.65 (0.71–3.88) | 0.248 |
| Extended to >1 cervical level | 2.12 (1.08–4.50) | 0.049c) |
| Serum CRP >20 mg/dL | 3.79 (1.80–7.98) | <0.001c) |
| Clinical outcomes and treatment response | ||
| Length of hospitalization >14 days | 4.10 (1.91–8.83) | <0.001c) |
| No. of surgical drainage >1 | 1.22 (0.60–2.59) | 0.579 |
| Death | 1.52 (0.13–5.99) | 0.833 |
| 1-Week follow-up CRP >5 mg/dL | 3.78 (1.76–8.10) | 0.001c) |
| Time interval for 50% decrease of initial CRP >5 days | 2.70 (1.15–6.34) | 0.023c) |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download