Abstract
Objectives
The pathophysiological mechanisms of chronic rhinosinusitis with nasal polyposis (CRSwNP) still are discussed controversially. Regulatory B cells (Breg) are responsible for the suppression of T cell activity: deficiencies for Breg have been demonstrated to contribute to autoimmune disorders, e.g., systemic lupus erythematosus. In order to evaluate the influence of B cell subpopulations, especially Breg, on the etiology of this disease, the aim of this study was to characterize subpopulations of peripheral and edaphic B cells in CRSwNP.
Methods
Polypoid tissue and blood samples were collected from 10 patients undergoing paranasal sinus surgery and lymphocytes were analyzed by multicolor flow cytometry.
Results
There was a significantly lower frequency of B cells in nasal polyps compared to peripheral blood mononuclear cells (PBMC) in patients with CRSwNP. Mature resting B cells were the main population within B cells in PBMC, and memory B cells in nasal polyps. Remarkably, Breg and mature B cells significantly decreased in nasal polyps compared to PBMC. Memory B cells significantly increased and represented the main subpopulation in nasal polyps in patients with CRSwNP.
The development of nasal polyps subdivides the chronic rhinosinusitis (CRS) into two different phenotypes: CRS with nasal polyposis (CRSwNP) and CRS without nasal polyposis (CRSsNP). Up to date, the etiopathology of CRSwNP is still not fully understood: nasal polyps develop from nasal mucosal tissue of the medial nasal concha and the superior nasal passage [1,2]. Histopathologically, they are characterized by respiratory epithelium and edematous stroma, which is infiltrated by a multitude of inflammatory cells. With respect to European and U.S. patients, the main cell populations are eosinophilic and lymphatic cells with a predominant TH2 subtype, whereas neutrophil inflammatory cells and a T helper 1 (TH1)/TH17 subtype are predominant in Asian patients [3]. Newly, Bachert et al. [3] developed a classification of CRSwNP into different endotypes depending on the predominant inflammatory cells in a TH2-biased or a TH1/TH17-biased inflammation. This is of clinical interest due to the higher risk of disease recurrence and bronchial asthma comorbidity in the TH2 endotype together with high levels of interleukin (IL)-5 and immunoglobulin E (IgE). According to Bachert et al. [3], a predominance of CD8+ T cells was shown in nasal polyps with a more effector memory phenotype in both CD4+ and CD8+ T cells in nasal polyps compared to peripheral blood mononuclear cells (PBMC) in patients with CRSwNP. Furthermore, among CD4+ T cells, activated regulatory T cells were increased in nasal polyps compared to PBMC [4]. Although T cells are known to be the predominant fraction of lymphocytes in polyps, other subpopulations like B cells can also be found in nasal polyps [5].
B cells are antigen-presenting cells and contribute to activating T cell help. Activated T cells interact with B cells under the influence of costimulatory molecules and cytokines, and induce signals for proliferation, survival, affinity maturation and isotype-switching. Some investigators have shown an upregulation of B and T cell interactions in nasal polyposis [6,7]. A special aspect of nasal polyps in comparison to infected nasal tissue in CRSwNP is a very high production of IgE [8]. These high local IgE levels differ from serum IgE levels in patients with CRSwNP, which underscores the importance of local IgE production [9]. Furthermore, specific IgE antibodies against Staphylococcus aureus enterotoxins were increased in nasal polyps and have a predictive value for concomitant asthma bronchiale [10]. These findings highlight the importance of local B cells in chronic infectious diseases of the nasal mucosa. They are fundamental to a variety of new-targeted approaches in CRSwNP therapy with very promising prospects [3].
Recently, immunological studies described a classification of B cells into regulatory B cells (Breg), mature B cells, memory B cells, plasma blasts and plasma cells. This subdivision is based on the expression of specific surface markers like CD19, CD24, CD38, CD27, CD20 and HLA-DR [11-14]. Breg are characterized as CD19+ CD20+ CD38high CD24high B cells. In comparison, mature B cells intermediately express the surface markers CD38 and CD24, whereas memory B cells are CD19+ CD20+ CD38– CD24high B cells. For identifying these subpopulations and analyzing the differences between peripheral and edaphic B cells, fluorescence-activated cell sorting (FACS) analysis is the method of choice [7,15,16]. Some investigators have shown dysregulations in the differentiation of B cells in autoreactive plasma cells, which can be responsible for autoimmune diseases [11]. Blair et al. [12] demonstrated an inhibition in the differentiation of TH1 cells in vitro by Breg isolated from human peripheral blood. However, this suppressive effect is lacking in patients with systemic lupus erythematosus (SLE). In addition, a correlation between the increase in CD19+ B cells and CD27high plasma cells and the activity of the disease in SLE is currently being discussed in the literature [11]. The aim of this study was to compare subpopulations of B cells in peripheral blood and nasal polyps of patients with CRSwNP using an up-to-date panel of markers for B cell subsets, particularly Breg, in order to elucidate their influence on the etiology of this disease.
This study was approved by the Ethics Board of the Medical Faculty, Julius-Maximilian-University Wuerzburg (vote 12/06), and all participants gave written informed consent.
Ten milliliters of heparinized blood samples were obtained intraoperatively by venous puncture from 10 patients undergoing paranasal sinus surgery and transferred to the laboratory. Lymphocytes were separated by density-gradient centrifugation (10 minutes, 1,000×g) at room temperature on equal amounts of Ficoll (Biochrom, Berlin, Germany), using a membrane-containing 10 mL cell tube (Greiner Bio-One, Frickenhausen, Germany). After washing twice in phosphate-buffered saline (PBS; Gibco BRL Life Technologies, Eggenstein, Germany), the cell number and the cell viability were determined using a Cell Counter+Analyser System (CASY TT; Innovatis, Reutlingen, Germany) according to the manufacturer’s protocol. After centrifugation with 1,600 rpm cells were frozen in –80℃ with 1 mL freezing medium, containing 10 parts fetal calf serum (Linaris, Dossenheim, Germany) and one part DMSO (Roth, Karlsruhe, Germany).
All tissue samples were collected intraoperatively from 10 patients undergoing standard paranasal sinus surgery. Additionally, two samples of normal nasal tissue as control group, where collected from two patients undergoing functional septorhinoplasty or uncinectomy because of a maxillary sinus cyst. The polyps and the nasal mucosae were cut into small fragments and mashed through a cell strainer (Greiner Bio-One) from 100 μm to 40 μm in PBS (Gibco). After washing twice in PBS, the cell number and cell viability were determined using a CASY system according to the manufacturer’s protocol. After centrifugation (5 minutes, 1,600 rpm) cells were frozen at –80℃ with 1 mL freezing medium.
The following antibodies were used: anti-CD45 Pacific Orange, anti-CD19 Brilliant Violet, anti-CD20 PE, anti-CD38 antigen-presenting cells, anti-CD24 PE.Cy7, anti-CD27 Per.CP-Cy5.5 and anti-HLA-DR Alexa 700 (all by BioLegend, San Diego, CA, USA). Viability Dye 780 (eBioscience, San Diego, CA, USA) was used to detect apoptotic cells. After blocking with 25 μg/mL normal mouse IgG (Sigma-Aldrich, St. Louis, MO, USA) for 15 minutes on ice, all cells underwent cell surface staining. All antibodies were used according to the manufacturers’ instructions. FACS analyses were performed using an LSR II flow cytometer (Becton Dickinson, San Diego, CA, USA), and the data were analyzed with FlowJo software (TreeStar, Ashland, OR, USA).
Data are presented as mean±standard deviation. Statistical significance was analysed by a two-tailed paired t-test using GraphPad Prism Software 6.0c (GraphPad Software Inc., La Jolla, CA, USA). For nonparametric distribution the Wilcoxon test was applied. The P-values <0.05 were considered statistically significant.
Ten patients were included in the study group (six males and four females). The mean age was 56±13 years. Inclusion criteria were a chronic rhinosinusitis with nasal polyps and the failure of medical treatment. Patients with established immunodeficiency, pregnancy, coagulation disorder, classic allergic fungal sinusitis, isolated antrochoanal polyps or cystic fibrosis were excluded from the study. All patients received intranasal topical steroids prior to the surgery according to the European guidelines [17]. This treatment started weeks before surgery and if the patients did not benefit from this medical therapy a surgical intervention was planned. Eosinophilic polyposis was described in the histological evaluation in most of the patients (8 of 10). Notably, in one histological finding with no eosinophilic infiltration numerous plasma blasts and plasma cells were visible. The characteristics are summarized in Table 1. Additionally, healthy nasal mucosa was obtained from two patients undergoing functional septorhinoplasty or uncinectomy because of a maxillary sinus cyst. The median age of these patients was 47±35 years.
Lymphocytes of nasal polyps and peripheral blood were analysed from 10 patients by flow cytometry by gating on CD45+ cells (Fig. 1A) after eliminating all apoptotic cells (Fig. 1B). Then, B cells were identified by gating on CD19+ and CD20+ cells (Fig. 2A) and B cell subpopulations by gating on CD38 and CD24 (Fig. 2B). The 6.5% of all lymphocytes in nasal polyps were CD19+ CD20+ B cells (Table 2). The frequency of B cells in nasal polyps was significantly lower than in PBMCs (Fig. 3A) and B cell subpopulations in nasal polyps differ from PBMCs (Fig. 3B). In healthy nasal mucosa frequencies of B cells (4.77±0.93) were decreased compared to PBMCs and nasal polyps. CD19+ CD20– plasma cells were significantly higher in nasal polyps compared to PBMC and in patients with CRSwNP (Table 3) and also compared to healthy nasal tissue (3.83±2.59). There was no significant difference between CD19+ CD20– CD27high HLA-DRhigh plasma blasts and CD19+ CD20– CD27high HLA-1,58DRlow plasma cells (Table 3, Fig. 4). Compared to nasal polyps and PBMCs, the lowest frequencies of CD19+ CD20– CD27high HLA-DRhigh plasma blasts and CD19+ CD20– CD27high HLA-DRlow plasma cells were found in healthy nasal mucosa (plasma blast, 2.49±1.48; plasma cells, 37.60 ±8.20).
CD19+ CD20+ CD38high CD24high Breg (Fig. 2B) were significantly lower in nasal polyps compared to PBMC in patients with CRSwNP (Table 2), but in both groups they were the minor subpopulation with only a few cells (Fig. 3B). In healthy mucosa no Breg were detected. Mature B cells described as CD19+ CD20+ CD38int CD24high B cells (Fig. 2B) were significantly lower in nasal polyps compared to peripheral blood (Table 2, Fig. 3B). In nasal mucosa the lowest frequencies of mature B cells (21.20±4.24) were measured. In PBMCs, mature B cells (Fig. 2B) were the highest frequency subpopulation (Fig. 3B). CD19+ CD20+ CD38– CD24high memory B cells (Fig. 2B) were the main subpopulation of B cells in nasal polyps and in healthy nasal mucosa (46.60±1.31). Memory B cells significantly increased in nasal polyps compared to PBMCs (Table 2, Fig. 3B).
In this study, a detailed quantification of subpopulations of peripheral and edaphic B lymphocytes in CRSwNP is presented. The majority of lymphocytes in polyp tissue are T cells, particularly CD8+ cytotoxic T cells [18]. Furthermore, B cells may also play an important role in the pathogenesis of nasal polyps. Some investigators have already shown a possible pathogenic role of B cells in CRS via the inflammatory reaction of mucosal nasal tissue. Kato et al. [19] described increased levels of the B cell activating factor of the tumor necrosis factor family (BAFF) in CRSwNP, IL-6 and B-lymphocyte chemoattractant (CXCL13) [20-22], and a production of BAFF by epithelial cells. In the present study, a significantly lower frequency of CD19+ CD20+ B cells was found in nasal polyps compared to PBMCs in patients with CRSwNP. Only 6.5% of edaphic lymphocytes in nasal polyps were B cells. Among the B cell subpopulations, Breg and mature B cells were significantly lower in polypoid tissue. Memory B cells were significantly higher and represented the main subpopulation in nasal polyps compared to peripheral blood in patients with CRSwNP. In the present study, immunoglobulin-secreting plasma cells displayed a significantly higher frequency in nasal polyps compared to PBMC. Previous studies have also shown an increase in plasma cells as well, and a differentiation of B cells in nasal polyps [8] with high local production of IgE without higher serum IgE levels [9]. Memory B cells represent antigen-experienced B cells and are related to age: Weksler [23] showed changes in the B cell reservoir in elderly patients, which may contribute to their increased responsiveness to infections [24]. In patients with SLE, Jacobi et al. [14] found no correlation between frequency and total number of memory B cells and disease duration. Furthermore, they showed a statistically significant reduction in naïve and memory B cells, but no influence of immunosuppressive therapy on plasma cells in SLE. In the European Position Paper on Rhinosinusitis and Nasal Polyps [17], a surgical treatment of CRSwNP is only indicated after failure of medical treatment and if a local therapy with corticoids does not lead to an improvement in symptoms score. Patients included in the current study received topical or systemic corticosteroids preoperatively to reduce the size of nasal polyps according to the treatment guidelines of the university hospital. Therefore, it was not possible to include a control group without any pretreatment with corticosteroids. It is very likely that this therapy may have influenced the distribution of B cell subpopulations. A study design comparing patients with and without preoperative local or systemic immunosuppressive therapy would be of great interest.
Reduced numbers and/or function of regulatory T cells were demonstrated to be responsible for different types of autoimmune disorders and chronic infections [25]. Blair et al. [12] recently showed a similar population within B cells with regulatory functions (Breg). They also demonstrated that Breg could suppress TH1 differentiation by secretion of IL-10 when anti-CD80 and CD86 mAb are added in vitro. In SLE, B cells isolated from peripheral blood lacked this suppressive effect. In the present study, a statistically significant decrease in Breg in nasal polyps compared to peripheral lymphocytes was shown. Future studies should focus on the functional influence of Breg with respect to the immunosuppressive effects on T helper cells as in SLE [12].
As previously mentioned, autoreactive B cells play an important role in SLE, and preliminary attempts have been made to use B cell subset composition as a marker for disease activity [14]. Based on these findings, Tan et al. [26] attempted to examine autoreactive B cells in CRSwNP. They found production of autoantibodies in nasal polyps and concluded that these autoantibodies lead to autoimmunity. Furthermore, the investigators hypothesized that autoreactive memory B cells persist in inflammatory nasal tissue after surgery and are responsible for recurrence of nasal polyps. High frequencies of memory B cells in the present study suggest that B cell autoreactivity might be important for the development of CRSwNP. In the present study, the total number of B cells was significantly lower than in PBMCs. Although the total number of edaphic B cells was low in our observations, data from the literature indicates a key role of B cells in the maintenance of CRSwNP [8-10,20].
A detailed characterization of B cell subpopulations, such as in the present study, is necessary to gain further insight into the pathophysiology of nasal polyps. The development of targeted therapies addressing CRS requires a comprehensive overview on the immunological mechanisms of this disease. Increased levels of local IgE in nasal polyps have already led to the introduction of Omalizumab as an anti-IgE treatment in clinical trials [27]. Other B cell-directed therapies, e.g., rituximab in autoimmune disease, are also promising [28], and future studies should focus on special targets like anti-dsDNA or BAFF, which are already being investigated in clinical trials in SLE [26].
The small number of subjects limits the results of this study. A control group of healthy nasal mucosa was tried to establish. But, one problem of analysis of B cells of healthy mucosa is the very small amount of cells, so a meaningful statistical analysis is difficult. For multicolour FACS analysis more cells are necessary in the opinion of the authors. Frequencies of two samples are shown in this study to show the difference between the groups. The study is declared to be a pilot project with a limited amount of samples. Since there is no statistically significant control group with healthy mucosa, interpretation of the data is affected. For example, a significantly amount of Breg in nasal polyps compared to PBMC in patients with CRSwNP was observed. But, if the number of Breg is much lower in healthy control tissue than in polyps this would indicate that there is an accumulation of Breg in polyps. Without a control group of healthy mucosa, it is difficult to provide further mechanistic insight. Unfortunately, analysis of B cells from healthy nasal mucosa was tried to perform but failed due to low amounts of these cells in this noninflammatory tissue, for example only a total number of 100 B cells were found in the first sample of healthy mucosa. In the opinion of the authors, high cell amounts are necessary for this multicolour flow cytometry analysis and for a significant analysis of this data. When assuming that most of the lymphocytes in healthy nasal mucosa were circulating intravascular cells, a control group of PBMC seemed to be equivalent, and, furthermore, a comparison of isolated T cells from tissue with PBMC is well established in the literature [4,29,30].
In this study, a detailed contemporary characterization of B cell subpopulations is presented. Memory B cells are the main subpopulation in nasal polyps. Breg and mature B cells are significantly decreased in nasal polyps compared to PBMC in CRSwNP. Breg are necessary to suppress the T helper cell activation and T and B cell interaction. The question arises as to whether the reduced Breg frequencies cause T cell dysfunction and/or the high frequencies of memory B cells. Such principle pathophysiological relationships need to be clarified in future studies and further functional studies are necessary to gain further insight into the genesis of CRSwNP [29,30].
▪ Memory cells present the main B cell subpopulations in nasal polyps.
▪ Regulatory B cells and mature B cells significantly decrease in nasal polyps compared to peripheral blood mononuclear cell.
▪ Reduced regulatory B cells frequencies or high frequencies of memory B cells may cause T cell dysfunction.
ACKNOWLEDGMENTS
This work was supported in part by a grant from the Deutsche Forschungsgemeinschaft (SFB/TR 124 FungiNet, project C6), Bonn, Germany.
REFERENCES
2. Andrews AE, Bryson JM, Rowe-Jones JM. Site of origin of nasal polyps: relevance to pathogenesis and management. Rhinology. 2005; Sep. 43(3):180–4.
3. Bachert C, Zhang L, Gevaert P. Current and future treatment options for adult chronic rhinosinusitis: focus on nasal polyposis. J Allergy Clin Immunol. 2015; Dec. 136(6):1431–40.

4. Ickrath P, Kleinsasser N, Ding X, Ginzkey C, Beyersdorf N, Hagen R, et al. Characterization of T-cell subpopulations in patients with chronic rhinosinusitis with nasal polyposis. Allergy Rhinol (Providence). 2017; Oct. 8(3):139–47.
5. Dutsch-Wicherek M, Tomaszewska R, Strek P, Wicherek L, Skladzien J. The analysis of RCAS1 and DFF-45 expression in nasal polyps with respect to immune cells infiltration. BMC Immunol. 2006; Mar. 7:4.
6. Zhang LP, Lin L, Zheng CQ, Shi GY. T-lymphocyte subpopulations and B7-H1/PD-1 expression in nasal polyposis. J Int Med Res. 2010; Mar-Apr. 38(2):593–601.

7. Dutsch-Wicherek M, Tomaszewska R, Lazar A, Strek P, Wicherek L, Kijowski J, et al. The presence of B7-H4+ macrophages and CD25+CD4+ and FOXP3+ regulatory T cells in the microenvironment of nasal polyps: a preliminary report. Folia Histochem Cytobiol. 2010; Dec. 48(4):611–7.

8. Gevaert P, Nouri-Aria KT, Wu H, Harper CE, Takhar P, Fear DJ, et al. Local receptor revision and class switching to IgE in chronic rhinosinusitis with nasal polyps. Allergy. 2013; Jan. 68(1):55–63.

9. Van Zele T, Gevaert P, Holtappels G, van Cauwenberge P, Bachert C. Local immunoglobulin production in nasal polyposis is modulated by superantigens. Clin Exp Allergy. 2007; Dec. 37(12):1840–7.

10. Bachert C, Zhang N, Holtappels G, De Lobel L, van Cauwenberge P, Liu S, et al. Presence of IL-5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma. J Allergy Clin Immunol. 2010; Nov. 126(5):962–8.e1-6.

11. Hoyer BF, Manz RA, Radbruch A, Hiepe F. Long-lived plasma cells and their contribution to autoimmunity. Ann N Y Acad Sci. 2005; Jun. 1050:124–33.

12. Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity. 2010; Jan. 32(1):129–40.

13. Odendahl M, Mei H, Hoyer BF, Jacobi AM, Hansen A, Muehlinghaus G, et al. Generation of migratory antigen-specific plasma blasts and mobilization of resident plasma cells in a secondary immune response. Blood. 2005; Feb. 105(4):1614–21.

14. Jacobi AM, Odendahl M, Reiter K, Bruns A, Burmester GR, Radbruch A, et al. Correlation between circulating CD27high plasma cells and disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 2003; May. 48(5):1332–42.
15. Robinson DS. The role of regulatory T lymphocytes in asthma pathogenesis. Curr Allergy Asthma Rep. 2005; Mar. 5(2):136–41.

16. Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009; Jun. 30(6):899–911.
17. Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012; Mar. 50(1):1–12.

18. Derycke L, Eyerich S, Van Crombruggen K, Perez-Novo C, Holtappels G, Deruyck N, et al. Mixed T helper cell signatures in chronic rhinosinusitis with and without polyps. PLoS One. 2014; Jun. 9(6):e97581.

19. Kato A, Truong-Tran AQ, Scott AL, Matsumoto K, Schleimer RP. Airway epithelial cells produce B cell-activating factor of TNF family by an IFN-beta-dependent mechanism. J Immunol. 2006; Nov. 177(10):7164–72.
20. Kato A, Peters A, Suh L, Carter R, Harris KE, Chandra R, et al. Evidence of a role for B cell-activating factor of the TNF family in the pathogenesis of chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2008; Jun. 121(6):1385–92.e1-2.

21. Patadia M, Dixon J, Conley D, Chandra R, Peters A, Suh LA, et al. Evaluation of the presence of B-cell attractant chemokines in chronic rhinosinusitis. Am J Rhinol Allergy. 2010; Jan-Feb. 24(1):11–6.

22. Peters AT, Kato A, Zhang N, Conley DB, Suh L, Tancowny B, et al. Evidence for altered activity of the IL-6 pathway in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2010; Feb. 125(2):397–403.e10.

24. Agematsu K, Nagumo H, Yang FC, Nakazawa T, Fukushima K, Ito S, et al. B cell subpopulations separated by CD27 and crucial collaboration of CD27+ B cells and helper T cells in immunoglobulin production. Eur J Immunol. 1997; Aug. 27(8):2073–9.

25. Belkaid Y, Tarbell K. Regulatory T cells in the control of host-microorganism interactions (*). Annu Rev Immunol. 2009; Apr. 27:551–89.
26. Tan BK, Li QZ, Suh L, Kato A, Conley DB, Chandra RK, et al. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2011; Dec. 128(6):1198–206.e1.

27. Gevaert P, Calus L, Van Zele T, Blomme K, De Ruyck N, Bauters W, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol. 2013; Jan. 131(1):110–6.e1.

28. Dorner T, Radbruch A, Burmester GR. B-cell-directed therapies for autoimmune disease. Nat Rev Rheumatol. 2009; Aug. 5(8):433–41.

29. Murray T, Fuertes Marraco SA, Baumgaertner P, Bordry N, Cagnon L, Donda A, et al. Very late antigen-1 marks functional tumor-resident CD8 T cells and correlates with survival of melanoma patients. Front Immunol. 2016; Dec. 7:573.

30. Rau M, Schilling AK, Meertens J, Hering I, Weiss J, Jurowich C, et al. Progression from nonalcoholic fatty liver to nonalcoholic steatohepatitis is marked by a higher frequency of TH17 cells in the liver and an increased TH17/resting regulatory T cell ratio in peripheral blood and in the liver. J Immunol. 2016; Jan. 196(1):97–105.
Fig. 1.
(A) Detection of CD45+ lymphocytes in peripheral blood mononuclear cells (PBMC) and nasal polyps in patients with chronic rhinosinusitis with nasal polyposis (CRSwNP). (B) Separation of apoptotic cells from CD19+ lymphocytes in PBMC and nasal polyps in patients with CRSwNP. SSC-A, side scatter area.
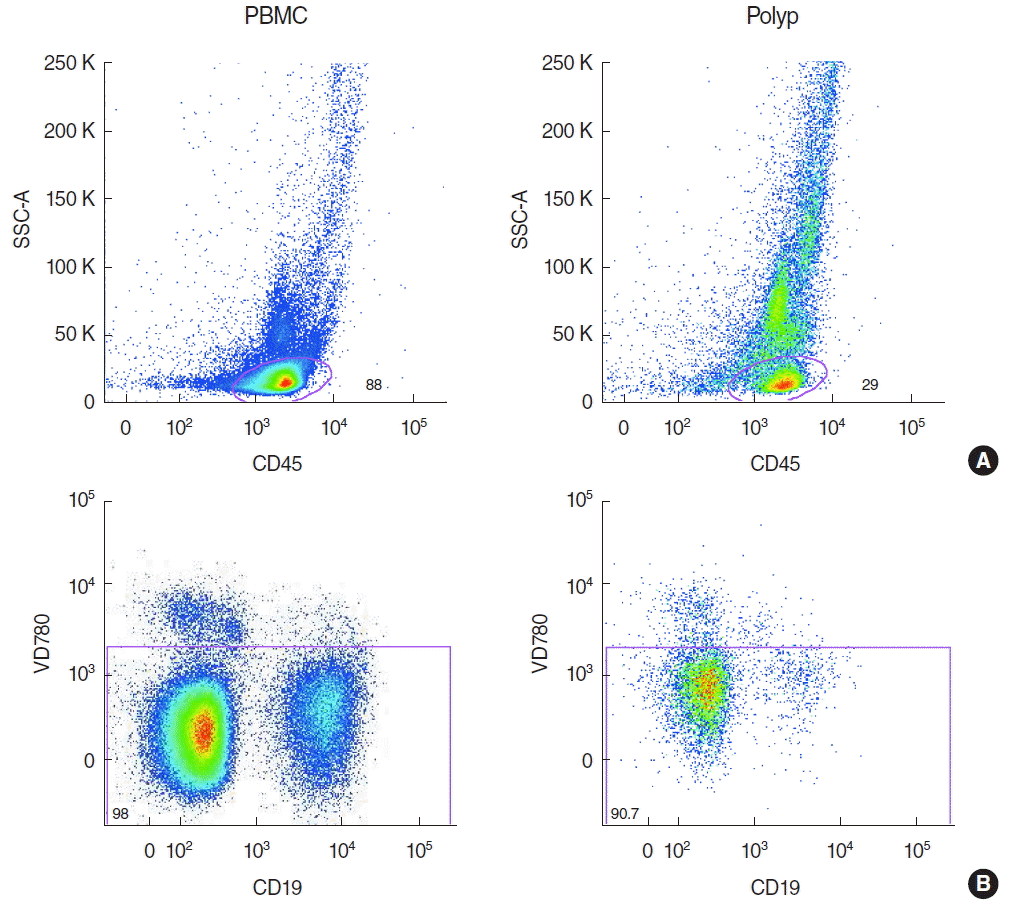
Fig. 2.
(A) CD19+ CD20+ B cells and CD19- CD20+ plasma cells in peripheral blood mononuclear cells (PBMC) compared to nasal polyps in patients with chronic rhinosinusitis with nasal polyposis (CRSwNP). (B) Differentiation of regulatory B cells, mature and memory B cells in PBMC and nasal polyps by gating on CD38 and CD24.
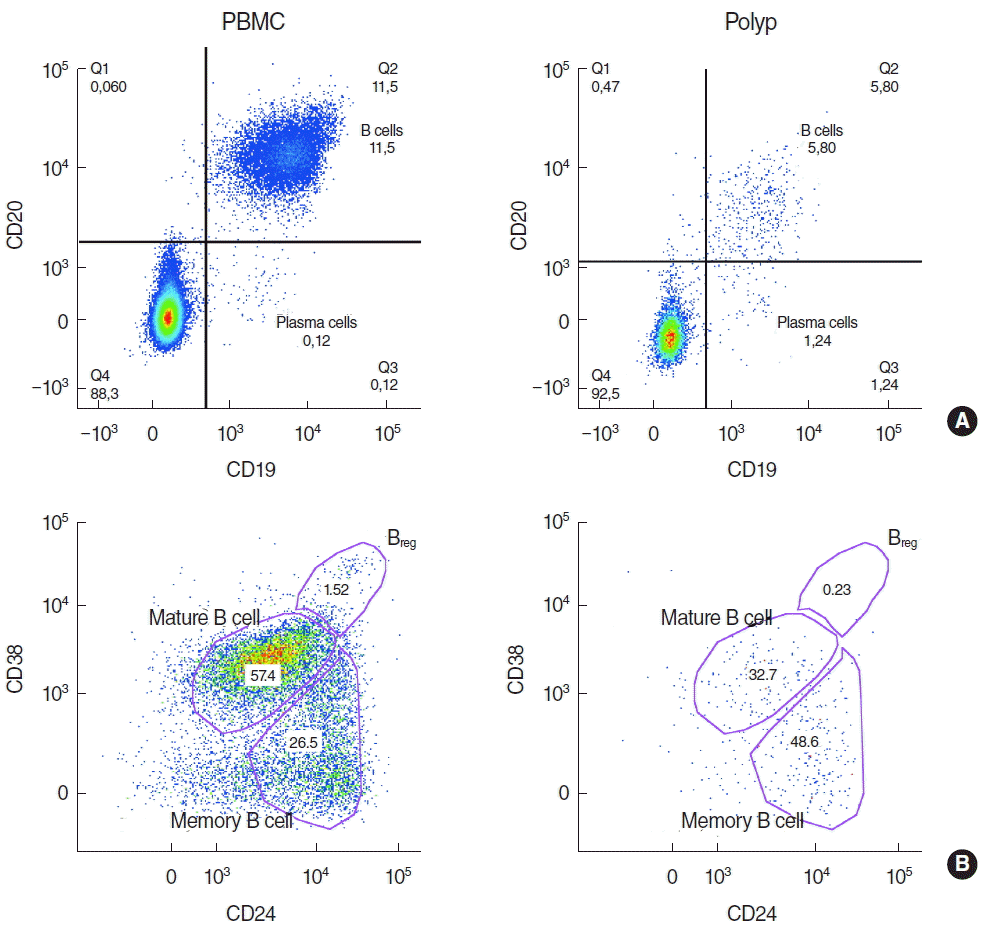
Fig. 3.
(A) Amount of B cells in nasal polyps compared to peripheral blood mononuclear cells (PBMC) in patients with chronic rhinosinusitis with nasal polyposis (CRSwNP). (B) Regulatory B cells (Breg), mature and memory B cells compared between lymphocytes from nasal polyps and PBMC in patients with CRSwNP.
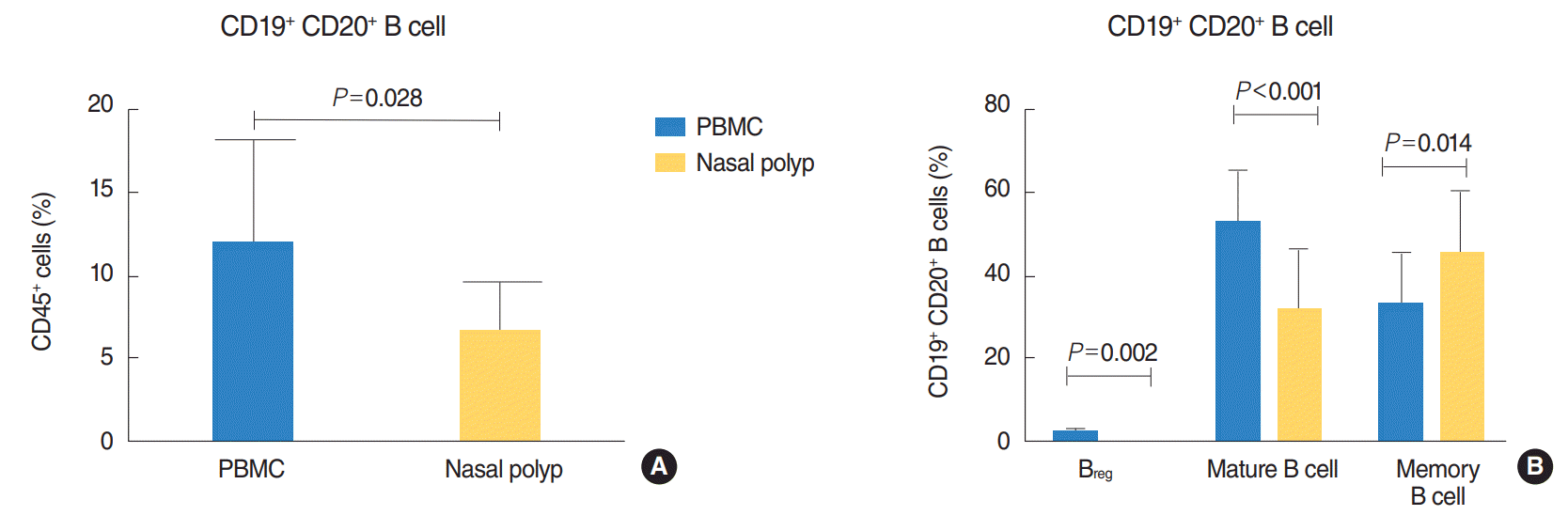
Fig. 4.
Characterization of CD19+ CD20- CD27high HLA-DRlow plasma cells and CD19+ CD20- CD27high HLA-DRhigh plasma blasts in peripheral blood mononuclear cells (PBMC) and nasal polyps in patients with chronic rhinosinusitis with nasal polyposis.
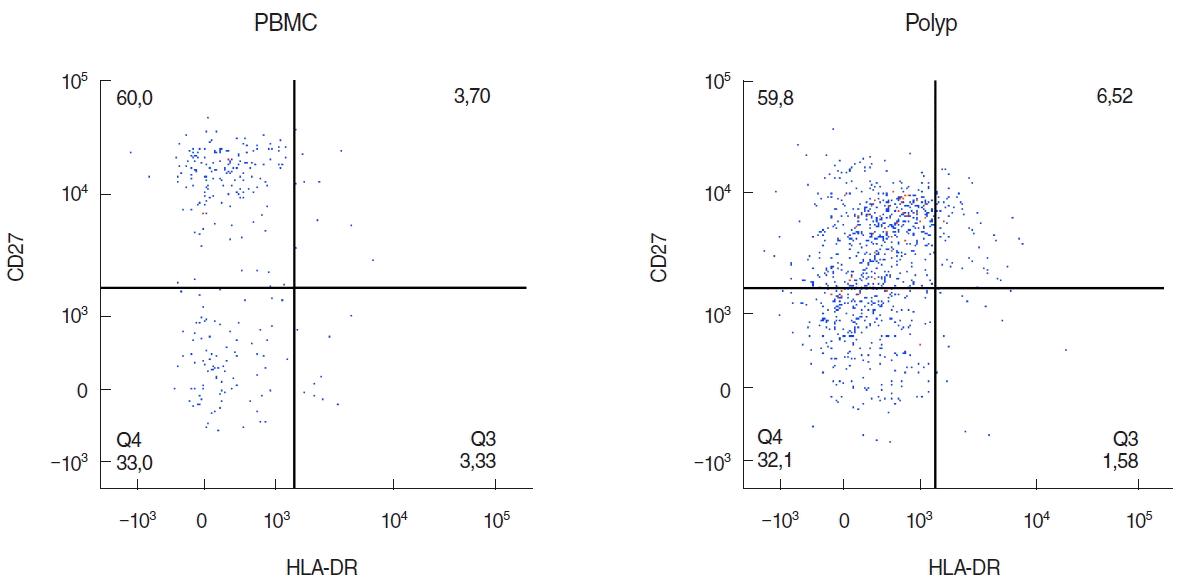
Table 1.
Demographic and clinical characteristics of the study group
| Characteristic | Value |
|---|---|
| No. of patients | 10 |
| Age (yr), mean±standard deviation | 56±13 |
| Female:male | 4:6 |
| Previous surgery | 3 |
| Eosinophilic polyp | 8 |
| Allergy | 4 |
| Samter’s triad | 1 |
Table 2.
B cell subpopulations analyzed by flow cytometry
| B cell | PBMCa) | CRSwNPa) | P-valuea) |
|---|---|---|---|
| CD19+ CD20+ B cell | 11.98±6.35 | 6.50±3.14 | 0.028 |
| CD38high CD24high Breg | 1.90±0.98 | 0.33±0.29 | 0.002 |
| CD38int CD24high mature B cell | 53.10±13.24 | 32.36±14.49 | <0.001 |
| CD38– CD24high memory B cell | 33.65±12.86 | 46.27±15.21 | 0.014 |




 Citation
Citation Print
Print


 XML Download
XML Download