Abstract
Objectives
Methods
Results
ACKNOWLEDGMENTS
REFERENCES
Fig. 1.
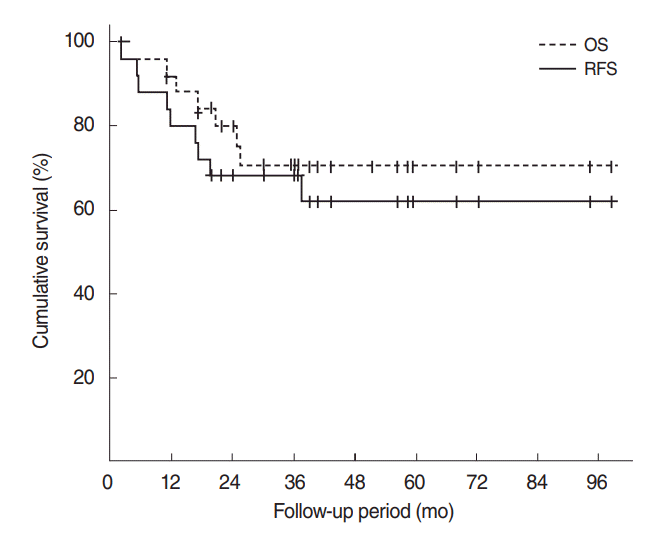
Table 1.
Table 2.
Table 3.
Table 4.
| Study | No. of cases | Staging system | Histologic grade | Treatment modality | Surgical procedure | Survival outcome | Analysis method | Prognostic/predictive factor | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Arriaga et al. (1989) [23] | 35 | PSS (1990) [3] | WD (14) | Surgery alone (3) | PTBR (21) | 2 yr OS: | NA | Prognostic factors associated with OS | |||
| T1 (4), T2 (1), T3 (19), T4 (15) | MD (17) | Surgery+PORT (32) | STBR (6) | T1, 2 (100%), T3 (50%), T4 (15%) | Margin status | ||||||
| PD (3) | TTBR (6) | T stage | |||||||||
| RM (2) | Dural involvement | ||||||||||
| Middle ear involvement | |||||||||||
| Lymph node involvement | |||||||||||
| Austin et al. (1994) [24] | 22 | PSS (1990) [3] | NA | Surgery alone (11) | LR (7) | 3 yr OS: | NA | NA | |||
| T1 (8), T2 (4), T3 (6), T4 (4) | Surgery+PORT (9) | PTBR (7) | S1 (60%), S2 (100%), S3 (50%), S4 (50%) | ||||||||
| Definitive RT (2) | STBR (4) | ||||||||||
| TTBR (2) | |||||||||||
| Hashi et al. (2000) [25] | 20 | Stell and McCormick (1985) [26] | NA | Surgery alone (0) | LR (4) | 5 yr OS: 59% | KM | NA | |||
| T1 (8), T2 (8), T3 (4), T4 (0) | Surgery+PORT (12) | PTBR (4) | |||||||||
| Definitive RT (8) | TTBR (5) | ||||||||||
| Moody et al. (2000) [1] | 32 | mPSS (2000) [1] | NA | Surgery alone (9) | LTBR (21) | 2 yr OS: | KM | Prognostic factors associated with OS | |||
| T1 (7), T2 (5), T3 (6), T4 (14) | Surgery+PORT (23) | STBR (6) | T1 (100%), T2 (80%), T3 (50%), T4 (7%) | Margin status | |||||||
| TTBR (5) | Advanced T stage | ||||||||||
| Dural involvement | |||||||||||
| Gillespie et al. (2001) [27] | 15 | mPSS (2000) [1] | NA | Surgery alone (6) | LR (3) | 2 yr OS: | NA | Stage | |||
| T1 (5), T2 (3), T3 (3), T4 (4) | Surgery+PORT (9) | LTBR (8) | T1, 2 (100%), T3 (50%), T4 (15%) | Extent of surgical resection | |||||||
| STBR (4) | |||||||||||
| Moffat et al. (2005) [6] | 39 | PSS (1990) [3] | WD (5) | Surgery alone (10) | LTBR (3) | 2 yr OS: | KM | Prognostic factors associated with OS | |||
| T1 (0), T2 (2), T3 (3), T4 (32) | MD (23) | Surgery+PORT (27) | ETBR (24) | T3 (50%), T4 (38%) | Lymph node involvement | ||||||
| PD (9) | Palliative RT (2) | RM (2) | 2 yr DFS: 43.2% | Poorly differentiated histology | |||||||
| Brain involvement | |||||||||||
| Salvage surgery | |||||||||||
| Yin et al. (2006) [12] | 95 | PSS (1990) [3] | NA | Surgery alone (17) | LR (23) | 5 yr OS: 66.8% | KM | Prognostic factors associated with OS | |||
| T1 (23), T2 (19), T3 (24), T4 (29) | Surgery+PORT (28) | PTBR (36) | S1, 2 (100%), S3 (67.2%), S4 (29.5%) | Log rank | Advanced stage | ||||||
| Definitive RT (16) | STBR (8) | Chi-square | Margin status | ||||||||
| Surgery+CRT (21) | |||||||||||
| RT+Chemo (12) | |||||||||||
| Surgery+Chemo (1) | |||||||||||
| Kunst et al. (2008) [14] | 28 | mPSS (2000) [1] | NA | Surgery alone (5) | LR (12) | 2 yr OS: 75% | KM | NA | |||
| T1 (12), T2 (2), T3 (4), T4 (10) | Surgery+PORT (23) | LTBR (11) | T1, 2 (85%), T3, 4 (64%) | ||||||||
| STBR (2) | 10 yr OS: 64% | ||||||||||
| TTBR (3) | T1, 2 (85%), T3, 4 (46%) | ||||||||||
| Lobo et al. (2008) [9] | 19 | PSS (1990) [3] | WD (10) | Surgery alone (5) | LTBR (11) | 5 yr OS: 37% | KM | Prognostic factors associated with OS | |||
| T1 (0), T2 (5), T3 (3), T4 (11) | MD (6) | Surgery+PORT (12) | STBR (1) | S2 (100%), S3 (25%), S4 (16%) | Lymph node involvement | ||||||
| PD (3) | Palliative RT (2) | TTBR (5) | 5 yr DFS: 37% | Facial palsy | |||||||
| S2 (100%), S3 (25%), S4 (16%) | |||||||||||
| Lassig et al. (2013) [4] | 30 | mPSS (2000) [1] | NA | Surgery alone (10) | LTBR (28) | 2 yr DFS: 70% | KM | Prognostic factors associated with DFS | |||
| T1 (4), T2 (3), T3 (6), T4 (14) | Surgery+PORT (16) | T1, 2 (100%), T4 (67%), T4 (56%) | Univariate | Advanced stage | |||||||
| Surgery+CRT (2) | Multivariate | Facial nerve resection | |||||||||
| Cox | Margin status | ||||||||||
| Adjuvant Chemo/RT | |||||||||||
| Essig et al. (2013) [15] | 35 | mPSS (2000) [1] | NA | Surgery alone (8) | LTBR (35) | 2 yr OS: 72% | KM | Prognostic factors associated with DSS | |||
| T1 (3), T2 (9), T3 (5), T4 (18) | Surgery+PORT (27) | 5 yr OS: 49% | Margin status | ||||||||
| 2 yr DFS: 68% | T stage | ||||||||||
| 5 yr DFS: 59% | |||||||||||
| 2 yr DSS: 79% | |||||||||||
| T1, 2 (100%), T3, 4 (70%) | |||||||||||
| 5 yr DSS: 62% | |||||||||||
| T1, 2 (100%), T3, 4 (45%) | |||||||||||
| Bacciu et al. (2013) [28] | 45 | mPSS (2000) [1] | WD (19) | Surgery alone (18) | LTBR (21) | 5 yr OS: 67.6% | KM | Prognostic factors associated with | |||
| T1 (5), T2 (6), T3 (15), T4 (19) | MD (23) | Surgery+PORT (27) | STBR (24) | 5 yr RFS: 68.9% | Univariate | DSS, RFS | |||||
| PD (3) | S1, 2 (100%), S3, 4 (59.6%) | Multivariate Cox | Advanced stage | ||||||||
| 5 yr DSS: 73.7% | Facial palsy | ||||||||||
| S1, 2 (100%), S3, 4 (61.5%) | Margin status | ||||||||||
| Fallopian canal involvement | |||||||||||
| Medial wall involvement | |||||||||||
| Middle ear involvement | |||||||||||
| Mastoid involvement | |||||||||||
| TMJ involvement | |||||||||||
| Jugular bulb involvement | |||||||||||
| Dural involvement | |||||||||||
| Leong et al. (2013) [8] | 35 | mPSS (2000) [1] | WD (9) | Surgery alone (0) | LTBR (10) | 2 yr OS: | KM | Prognostic factors associated with OS | |||
| T1 (4), T2 (1), T3 (3), T4 (27) | MD (18) | Surgery+PORT (35) | ETBR (25) | S1, 3 (100%), S4 (41.4%) | Poorly differentiated histology | ||||||
| PD (8) | 5 yr OS: 48.6% | ||||||||||
| Mazzoni et al. (2014) [7] | 41 | mPSS (2000) [1] | NA | Surgery alone (18) | LTBR (30) | DFS: T1, 2 (67%), T3, 4 (41%) | KM | Prognostic factors associated with | |||
| T1 (6), T2 (6), T3 (8), T4 (21) | Surgery+PORT (23) | STBR (11) | DSS: T1, 2 (92%), T3, 4 (48%) | DFS, DSS | |||||||
| Advanced stage | |||||||||||
| Lymph node involvement | |||||||||||
| Gandhi et al. (2016) [10] | 43 | Stell and McCormick (1985) [26] | WD (11) | Surgery alone (5) | STBR (4) | 2 yr OS: 50.7% | KM | Prognostic factors associated with PFS | |||
| T1 (2), T2 (17), T3 (18), unknown (6) | MD (12) | Surgery+PORT (11) | LTBR (2) | 2 yr PFS: 30.4% | Univariate | T stage (P =0.05) | |||||
| PD (1) | Definitive RT (14) | RM (10) | PFS for surgery+PORT vs. definitive RT: 85.7% vs. 46.9% | Multivariate | Facial palsy (P =0.0008) | ||||||
| NS (19) | Cox | ||||||||||
| This study | 26 | mPSS (2000) [1] | WD (16) | Surgery alone (11) | LR (3) | 5 yr OS: 70.4% | KM | Preoperative T stage | |||
| T1 (10), T2 (8), T3 (4), T4 (4) | MD (6) | Surgery+PORT (12) | LTBR (17) | 5 yr RFS: 61.8% | Univariate | Preoperative overall stage | |||||
| PD (1) | Surgery+Chemo (3) | ETBR (4) | |||||||||
| NS (3) | STBR (2) | ||||||||||
EAC, external auditory canal; PSS, University of Pittsburgh staging system proposed by Arriaga et al.; WD, well differentiated; MD, moderate differentiated; PD, poorly differentiated; PORT, postoperative radio therapy; PTBR, partial temporal bone resection; STBR, subtotal temporal bone resection; TTBR, total temporal bone resection; RM, radical mastoidectomy; OS, overall survival; NA, not available; RT, radiotherapy; LR, local resection; KM, Kaplan-Meier product-limit method; mPSS, modified University of Pittsburgh staging system proposed by Moody et al.; LTBR, lateral temporal bone resection; ETBR, extended temporal bone resection; DFS, disease free survival; CRT, chemoradiotherapy; Chemo, chemotherapy; DSS, disease specific survival; RFS, recurrence-free survival; TMJ, temporomandibular joint; PFS, progression free survival.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download