INTRODUCTION
MATERIALS AND METHODS
Chemicals and zebrafish preparation
Assessment of embryotoxicity in zebrafish
Assessment of hair cell toxicity in zebrafish
Measurement of intracellular reactive oxygen species generation
Scanning electron microscopy
Statistical analysis
RESULTS
Embryotoxicity of nicotine in zebrafish (brn3c and cmcl2)
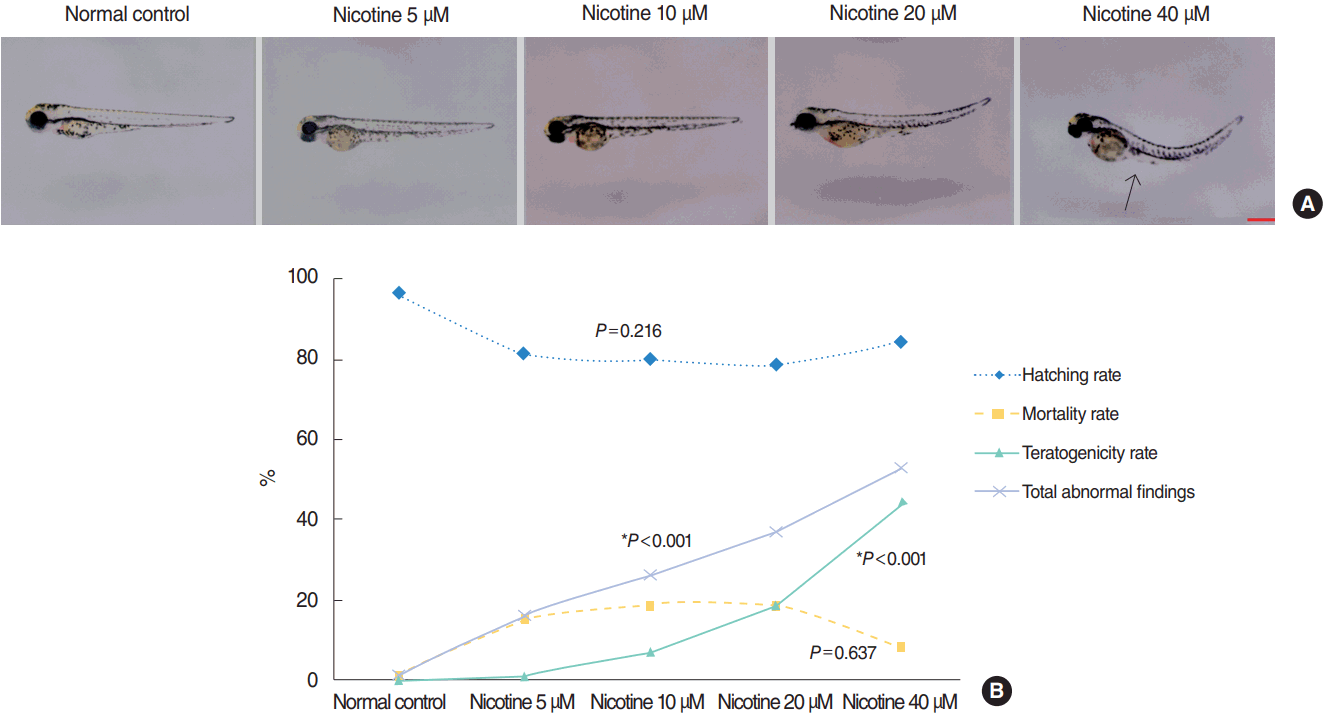 | Fig. 1.Nicotine-induced embryotoxicity at 72 hours post-fertilization (hpf). (A) Hatching/non-hatching embryo findings. The number of embryos showing abnormal morphology tended to be increased in the nicotine-exposed groups (arrow: bending of the trunk). All images were captured at 72 hpf (×32). Scale bar=1,000 μm. (B) Hatching rate, mortality rate, teratogenicity rate, and total abnormal findings (mortality rate+teratogenicity rate) showed statistically significant differences among nicotine concentrations (n=100 per concentration, linear by linear association). Nicotine-exposed groups showed a lower hatching rate and higher mortality and teratogenicity than the control group. The rate of teratogenicity and total abnormal findings increased in a dose-dependent manner (1% in the normal control group, 16% for 5 μM nicotine, 26% for 10 μM nicotine, 37% for 20 μM nicotine, and 53% for 40 μM nicotine; (total n=400). *Statistically significant. All data were evaluated at 72 hpf. |
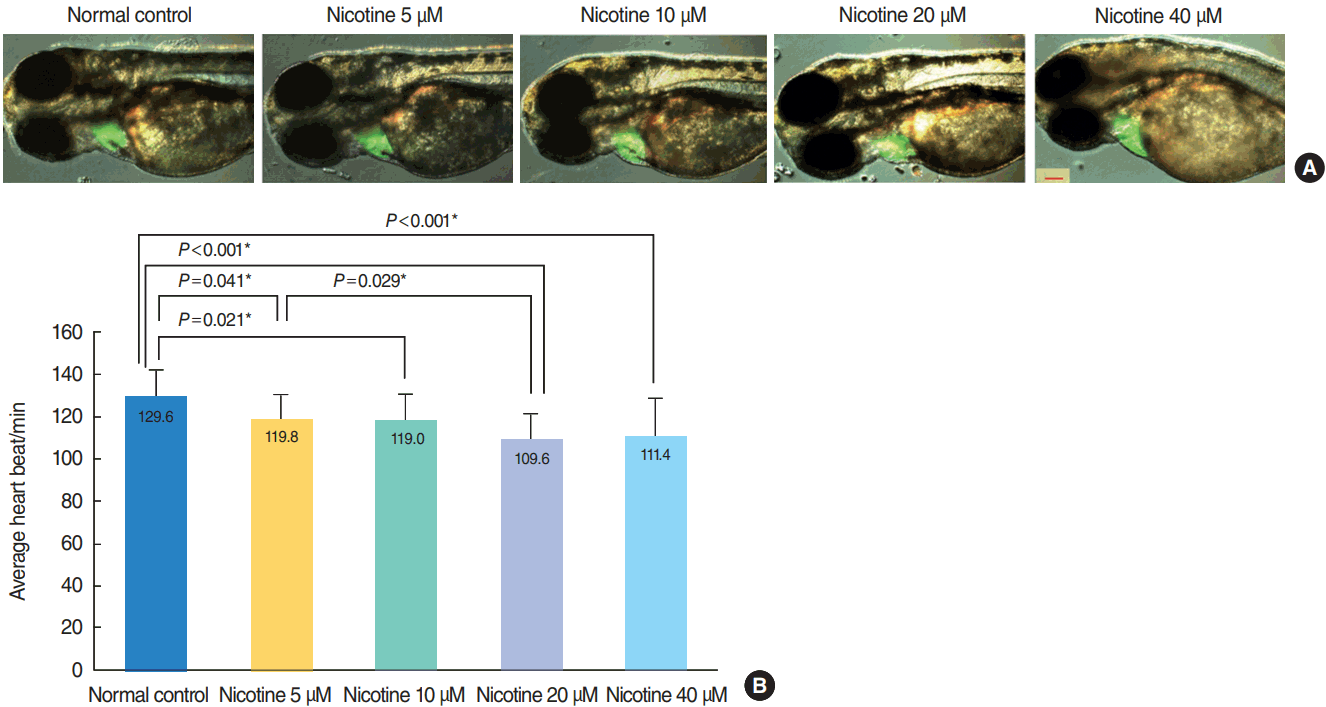 | Fig. 2.Heart morphology evaluation and changes in heart rate induced by nicotine treatment. (A) Merged images from the fluorescent image and optical image are shown. There was no difference in heart size between groups (green area). All images were captured using transgenic (cmlc2:EGFP) zebrafish at 72 hours post-fertilization (hpf, ×16). Scale bar=200 μm. (B) The heart rate of zebrafish embryos was significantly decreased in nicotine-exposed groups (P <0.001, one-way analysis of variance). All data were evaluated at 72 hpf. Only statistically significant pair-wise comparisons in post-hoc analysis are shown. *Statistically B significant (P<0.05, total n=150). |
Nicotine-induced hair cell toxicity in neuromasts of zebrafish embryos (brn3c)
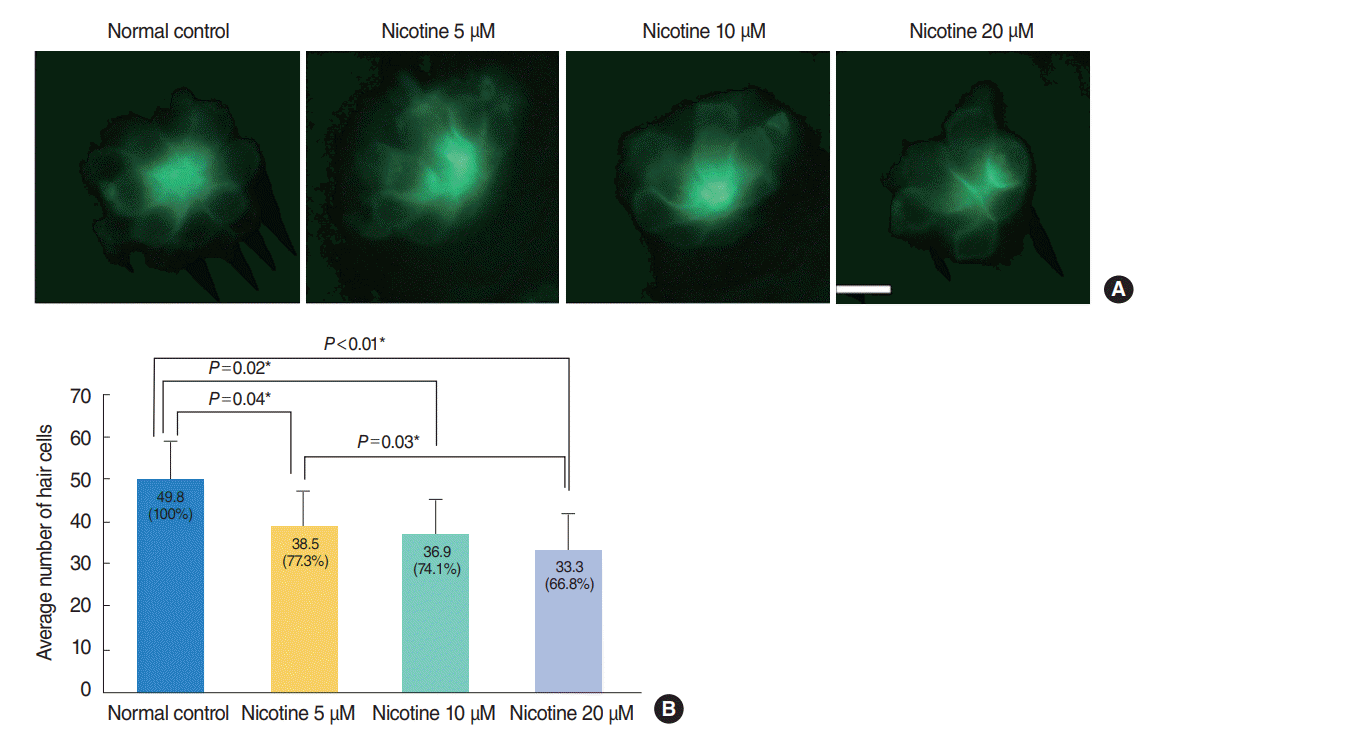 | Fig. 3.Nicotine-induced hair cell toxicity at 120 hours post-fertilization (hpf). The total number of hair cells was measured in four neuromasts (supraorbital [SO1 and SO2], otic [O1], and occipital [OC1]; total n=150). (A) The number of hair cells decreased in nicotine-exposed groups as shown by fluorescent microscopy (OC1, ×40). Scale bar=10 μm (×10). (B) Animals in the normal control group had an average of 49.8 hair cells. Nicotine exposure significantly decreased the number of total hair cells of the four neuromasts compared with that of the normal control, (P<0.001, one-way analysis of variance). At a concentration of 40 μM nicotine almost all the fish were dead, therefore statistical analysis was unavailable. All data were evaluated using transgenic (brn3c:EGFP) zebrafish at 120 hpf. Only statistically significant pair-wise comparisons in posthoc analysis are shown. *Statistically significant (P<0.05). |
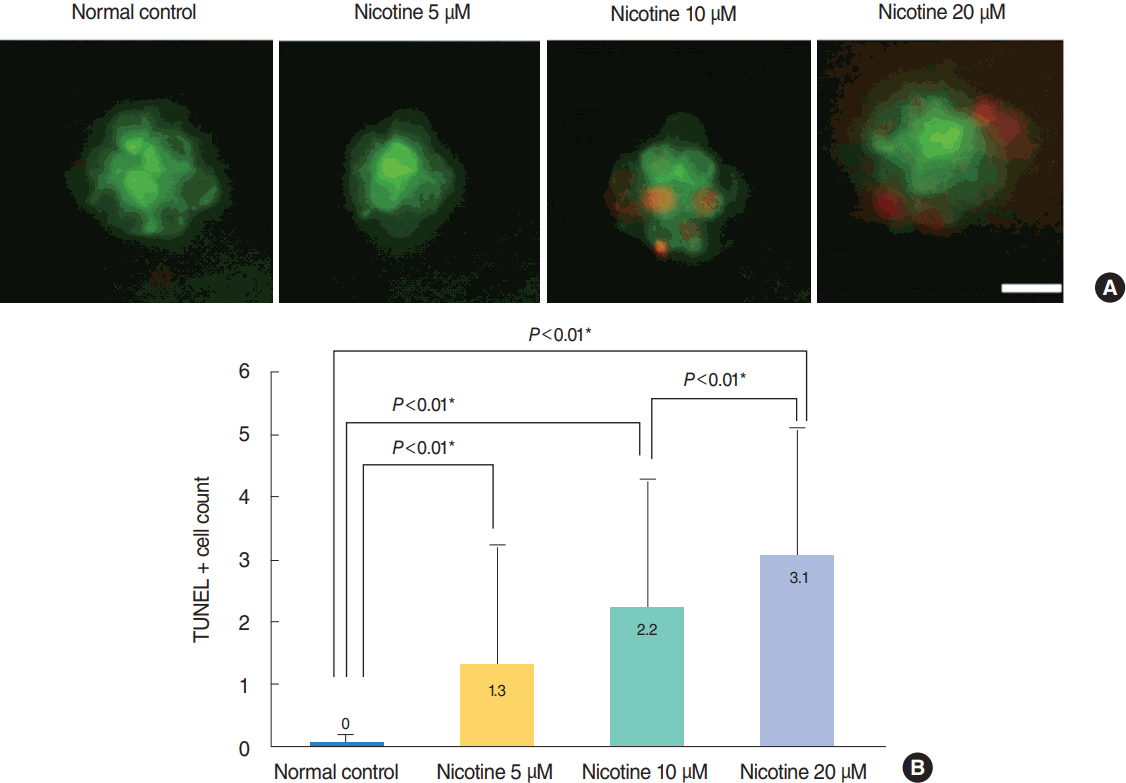 | Fig. 4.Nicotine-induced apoptosis in hair cells within neuromasts. Nicotine-induced apoptosis was confirmed by terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) assay. (A) Green dots represent hair cells. Apoptotic cells appear as light red dots in the merged image obtained by fluorescent microscopy after the TUNEL reaction. All images were obtained using transgenic (brn3c:EGFP) zebrafish at 120 hours post-fertilization. Scale bar=10 μm (×40). (B) The average number of TUNEL-positive cells was measured in each neuromast (supraorbital [SO1, SO2], otic [O1], and occipital [OC1]; total n=50). At a concentration of 40 μM, the fish were almost all dead and statistical analysis was unavailable. The number of TUNEL-positive cells was significantly increased in nicotine-exposed groups (P<0.001, one-way analysis of variance). Only statistically significant pair-wise comparisons in post-hoc analysis are shown. *Statistically significant (P<0.05). |
DASPEI staining in wild-type embryos
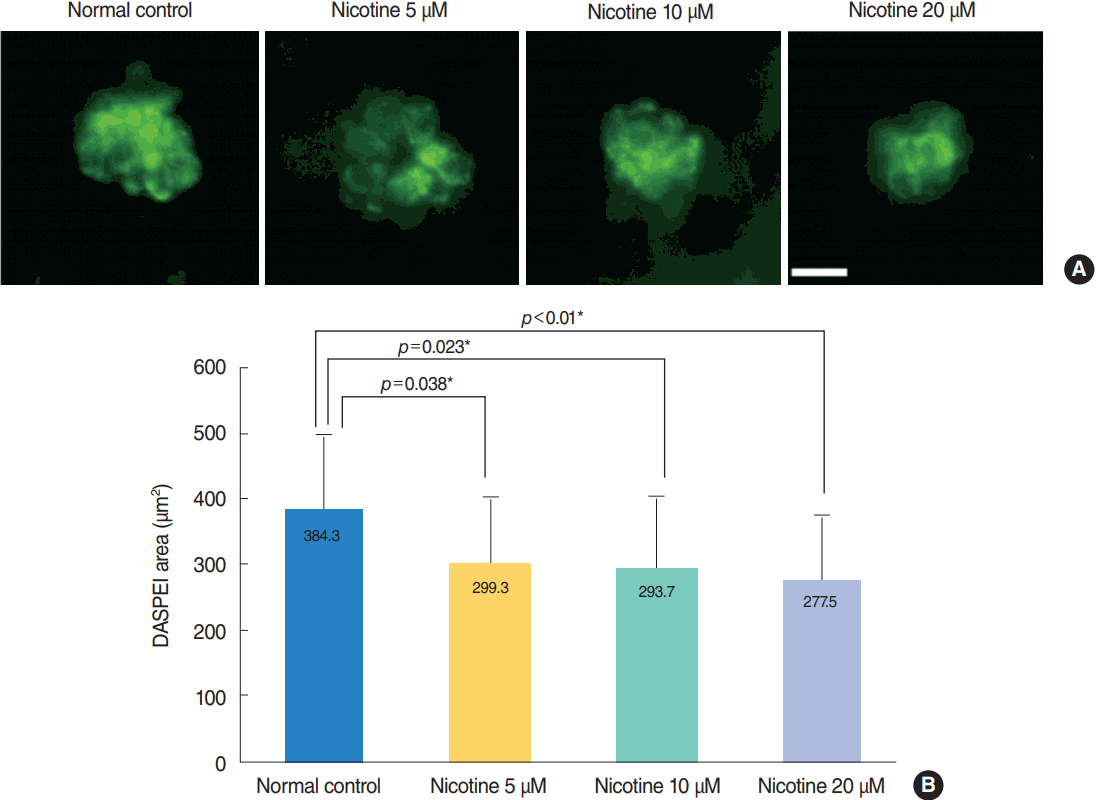 | Fig. 5.Analysis of hair cell damage by 2-(4-[dimethylamino]styryl)-N-ethylpyridinium iodide (DASPEI) assay. (A) Staining of hair cells with DASPEI showed that treatment with nicotine decreased the number of hair cells in neuromasts. Scale bar=10 μm (×40). (B) Average DASPEIstained area in four neuromasts (supraorbital [SO1, SO2], otic [O1], and occipital [OC1]; total n=30) significantly decreased as nicotine concentration increased (P<0.001, one-way analysis of variance). Fish exposed to 40 μM nicotine were almost all dead after 120 hours post-fertilization. Only statistically significant pair-wise comparisons in post-hoc analysis are shown. *Statistically significant (P<0.05). |
Measurement of intracellular ROS generation in wild-type larvae
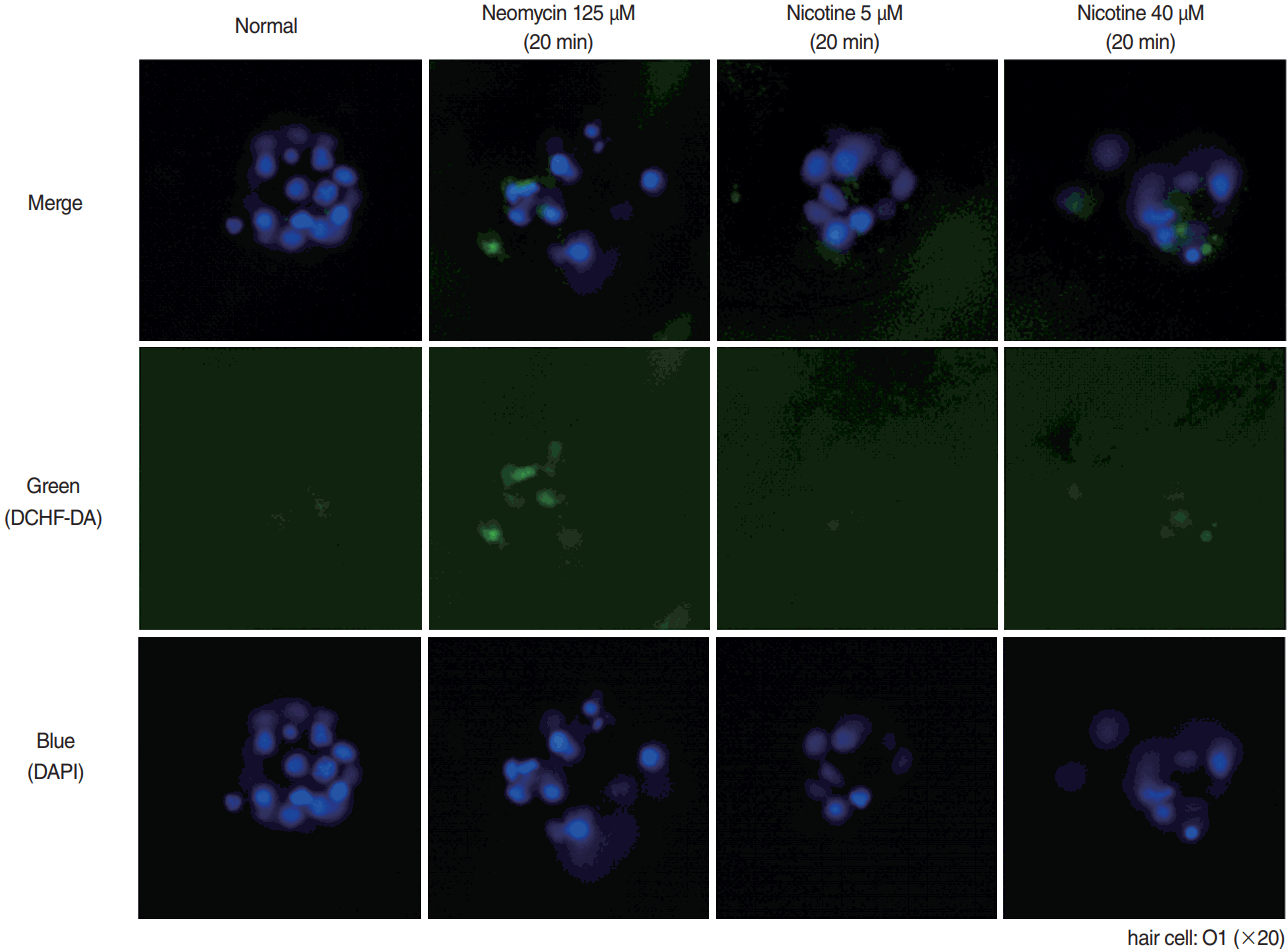 | Fig. 6.Reactive oxygen species (ROS) production by 2,7-dichlorodihydrofluorescein diacetate (DCHF-DA) assay. For evaluation of ROS generation, wild type zebrafish was used with DCHF-DA assay on hair cells as in green dots in the middle row. A 4´,6-diamidino-2-phenylindole (DAPI)-positive cells were observed as blue fluorescent dots as in the lower row. By merging those figures, the ROS production cells could be clearly identified as in the upper row. Neomycin as positive control showed strongest ROS generation. Despite not strong as in neomycin, there was increased ROS generation in hair cells in nicotine 40 μM. O1, otic. |
Nicotine-induced ultrastructural changes by SEM
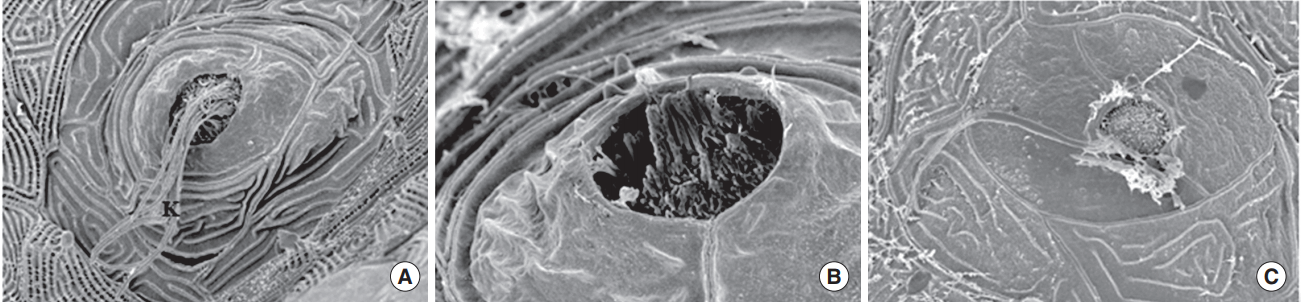 | Fig. 7.Scanning electron microscopy. When transgenic (brn3c:EGFP) zebrafish were exposed to nicotine for 120 hours, the kinocilia of hair cells in neuromasts were clearly observed in the normal control (A) and 5 μM nicotine group (B), but not in the 20 μM nicotine group (C). All images were obtained in three zebrafish at 120 hours post-fertilization for each group. Scale bar (at the bottom of each figure, one space)=5 or 10 µm. |




 Citation
Citation Print
Print


 XML Download
XML Download