Abstract
Objectives
The number of metastatic lymph nodes (LNs) and the ratio between the number of metastatic LNs and the total number of retrieved LNs (the LN ratio [LNR]) have been proposed as risk factors for recurrence of papillary thyroid carcinoma (PTC). However, the significance of the number of LNs and the LNR in patients with clinically node negative PTC has not been clearly determined. The purpose of this study is to evaluate their significance.
Methods
We retrospectively analyzed 382 patients with PTC who had undergone total thyroidectomy with prophylactic central neck dissection (CND) between January 2000 and December 2010. We excluded patients with lobectomy, concurrent lateral compartment neck dissection, a follow-up period less than at least 2 years, number of harvested central LNs less than or equal to one, clinically positive LN, distant metastasis, recurrent cancer or other types of malignancy. The correlations between recurrence and various clinicopathologic characteristics including tumor size, extrathyroidal extension (ETE), stage, number of metastatic central LNs, and the LNR were investigated.
Results
After a mean follow-up period of 82.2±26.4 months, recurrence occurred in 14 patients (3.7%). Tumor size ≥20 mm, maximal ETE, presence of central LN metastasis, number of metastatic LNs ≥2, and LNR ≥0.31 correlated with recurrence in the univariate analysis. However, tumor size ≥20 mm, maximal ETE, number of metastatic LNs ≥2, and LNR ≥0.31 were significantly associated with recurrence in the multivariate analysis (hazard ratio=6.61, 7.17, 3.43, and 11.23, respectively).
Go to : 
During the past 20 years, the incidence of thyroid cancer has increased sharply all over the world mainly due to increased detection using ultrasonography [1], and the incidence rate of thyroid cancer in Korea is the highest in the world [2]. The incidence of papillary thyroid carcinoma (PTC) accounts for the majority of this increase [3]. Although patients with PTC have excellent prognosis and survival, recurrence occurs in 5%–21% of patients with PTC [4,5]. Recurrence of PTC usually requires a second operation, which carries a higher complication rate, especially if central neck dissection (CND) is necessary. Therefore, the prevention of recurrence is important in the management of PTC to minimize treatment morbidity and improve both quality of life and survival rate.
Tumor size, extrathyroidal extension (ETE), age, lymph node (LN) metastasis, tumor multiplicity, extranodal spread, and male sex are all associated with increased risk of recurrence of PTC [6-8]. LN metastasis occurs in 20%–90% of patients with PTC [9-11], and is associated with increased recurrence and decreased survival in patients with PTC [12,13]. Recently, the number of metastatic LNs and the ratio of metastatic LNs to the total number of retrieved LNs (the LN ratio [LNR]) have been suggested as prognostic risk factors for recurrence in patients with PTC [14-21]. However, the clinical significance of these measures in patients with clinically node negative (cN0) PTC has not yet been established clearly. The aim of this study is to evaluate the prognostic significance of the number of metastatic LNs and the LNR of central compartment LNs in patients with cN0 PTC.
Go to : 
We reviewed retrospectively the data from 382 patients with PTC who underwent total thyroidectomy with prophylactic CND between January 2000 and December 2010. Bilateral CND was performed in 258 patients (67.5%) and unilateral CND in 124 patients (32.5%). We excluded patients who had clinically positive LN metastasis in preoperative work-up, who underwent lobectomy or concurrent lateral compartment neck dissection, or patients with a follow-up period of less than at least 2 years, the number of harvested LNs less than or equal to one, recurrent tumor, distant metastasis, other types of malignancy or history of thyroid surgery or irradiation. This study was approved by the Institutional Review Board of Hanyang University Hospital (IRB No. 2017-09-028) and the informed consent was waived. We conducted this study in compliance with the principles of the Declaration of Helsinki.
Radioactive iodine (RAI) ablation at a dose of 30 to 150 mCi was performed 2 to 4 months postoperatively. RAI ablation was performed in all patients with gross ETE, or a tumor size larger than 4 cm, or distant metastases. RAI ablation was also recommended for selected patients with minimal ETE or cervical LN metastasis and tumor size 1 to 4 cm and/or higher risk histologic features similar to American Thyroid Association guidelines [22].
Physical examination, neck ultrasonography, serum thyroid-stimulating hormone-stimulated or thyroid-stimulating hormone-suppressed thyroglobulin (Tg) measurements, and anti-Tg antibody measurements were used to detect recurrence of PTC after surgery at 6 to 12-month intervals. Computed tomography, whole-body iodine scan, or fluorodeoxyglucose-positron emission tomography-computed tomography were also used to detect recurrence if necessary. Recurrence was defined as the development of new abnormal structural lesions identified by one of the imaging methods mentioned previously, and was confirmed pathologically using fine needle aspiration cytology.
The whole thyroid gland and the central LNs removed were analyzed histopathologically to evaluate tumor characteristics, including tumor size, multiplicity, bilaterality, lymphatic or vascular invasion, ETE, the total number of LNs, and the number of metastatic LNs. The LNR was defined as the total number of metastatic LNs divided by the total number of LNs retrieved from the central compartment.
The cutoff values for the number of metastatic LNs and the LNR were determined when sensitivity and specificity were optimized using receiver operating characteristic curve analysis.
The correlation of recurrence of PTC with various clinicopathologic factors, including age, sex, tumor size, ETE, multiplicity, bilaterality, TNM (tumor, node, and metastasis) classification stage, number of metastatic LNs and the LNR was analyzed using Pearson’s chi-square test and Fisher exact test. Factors that were statistically significant in the univariate analysis were further analyzed by multiple logistic regression.
Recurrence-free survival (RFS) curves were calculated using the Kaplan-Meier method and compared using the log-rank test. A P-value less than 0.05 was considered to be statistically significant. All statistical analyses were performed using IBM SPSS ver. 21.0 (IBM Corp., Armonk, NY, USA).
Go to : 
The clinicopathologic characteristics are summarized in Table 1. The study included 55 men and 327 women, and the mean age was 47.8±12.5 years. The mean tumor size was 12.0±8.1 mm. Minimal ETE (e.g., tumor extension to the sternothyroid muscle or perithyroidal soft tissues) was found in 174 patients (45.5%), and maximal ETE (e.g., tumor extension to the trachea, esophagus, recurrent laryngeal nerve, larynx, pharynx, subcutaneous soft tissue, or skin) was found in 15 patients (3.9%).
Clinicopathologic characteristics and surgical outcome of the papillary thyroid carcinoma patients who underwent total thyroidectomy with prophylactic central neck dissection
Central LN metastasis was confirmed in 144 patients (37.7%) in the final pathologic report. The mean number of harvested and metastatic central LNs was 7.14±6.23 (range, 2 to 40) and 1.13±2.35 (range, 1 to 21), respectively, and the mean LNR was 0.18±0.30. Postoperative RAI ablation was performed in 289 patients (75.7%).
Of the 382 patients, 14 (3.7%) suffered recurrence during the mean follow-up period of 82.2±26.4 months. Seven patients had recurrence in the lateral compartment LN, six patients in the central neck, and one in a distant site (the lung). The patient with metastasis in the lung died from the disease.
The cutoff value for the number of metastatic LNs was two, with a sensitivity of 42.9% and a specificity of 79.3% (area under curve, 0.728; standard error, 0.069). The cutoff value for the LNR was 0.31, with a sensitivity of 71.4% and a specificity of 77.4% (area under curve, 0.776; standard error, 0.072) (Fig. 1).
The univariate and multivariate correlations between clinicopathologic characteristics and recurrence is presented in Tables 2 and 3, respectively. In the univariate analysis, tumor size ≥20 mm, maximal ETE, the presence of central LN metastasis, the numbers of metastatic central LNs ≥2, and a central LNR ≥0.31 were associated statistically with recurrence (Table 2).
Univariate analysis for factors related to recurrence in patients with clinically node negative papillary thyroid carcinoma (n=382)
Multivariate analysis for factors related to recurrence in patients with clinically node negative papillary thyroid carcinoma
In the multivariate analysis, tumor size ≥20 mm, maximal ETE, the number of metastatic LNs ≥2, and a central LNR ≥0.31 were associated statistically with recurrence, whereas the presence of central LN metastases showed no statistical significance (Table 3). The hazard ratios for the number of metastatic LNs ≥2 and central LNR ≥0.31 were 3.43 (95% confidence interval [CI], 1.04 to 11.26) and 11.23 (95% CI, 2.81 to 44.82), respectively.
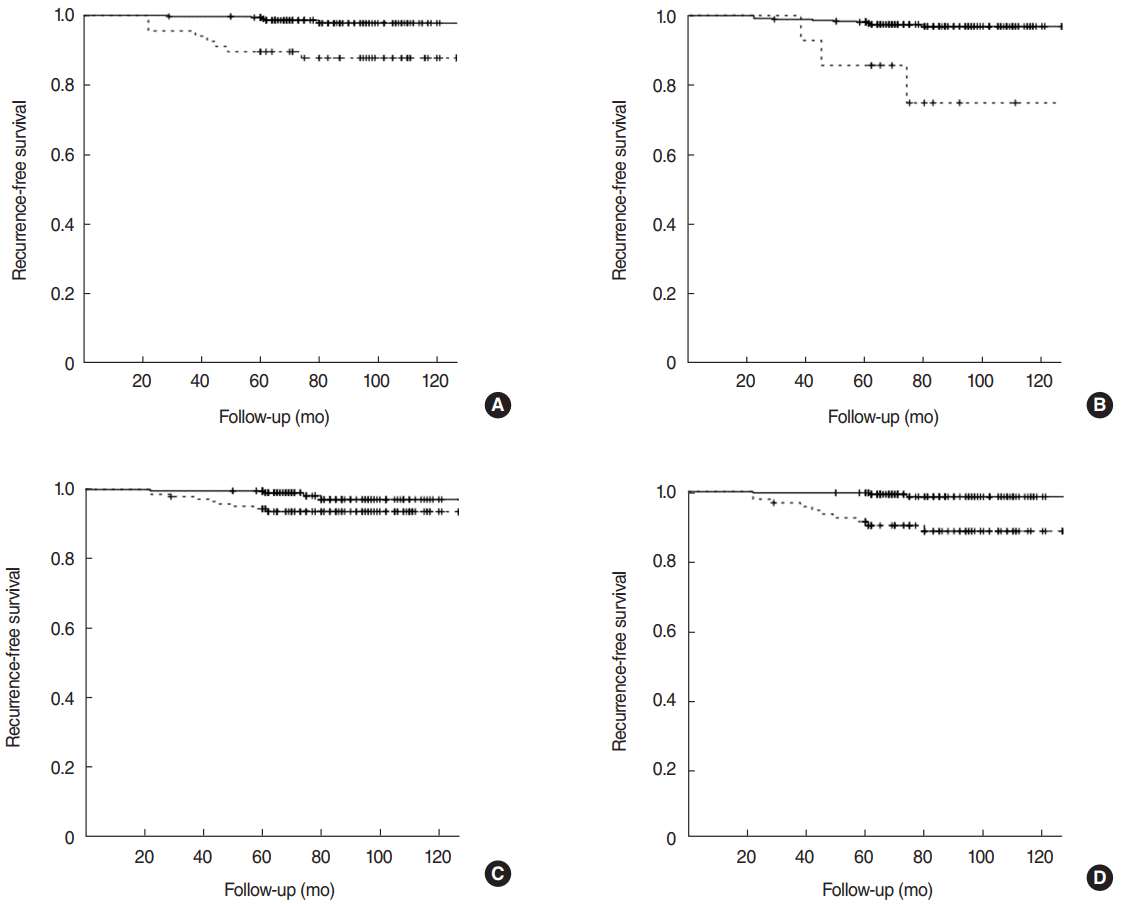
The 10-year RFS rate was 96.0%. The RFS rate was significantly decreased in patients with tumor size ≥20 mm (97.8% vs. 87.8%, P<0.001), maximal ETE (96.9% vs. 75.0%, P<0.001), number of metastatic LNs ≥2 (97.1% vs. 93.7%, P=0.025) and central LNR ≥0.31 (98.5% vs. 88.6%, P<0.001) (Fig. 2).
Go to : 
Prophylactic CND remains still controversial in patients with cN0 PTC. Argues against prophylactic CND include a lack of substantial evidence of the benefit and the potential morbidity of the procedure [11,23]. However, prophylactic CND may LN recurrence, and it provides more accurate staging for decisions regarding adjuvant RAI ablation [24,25]. The aim of this study was to evaluate the clinical significance of the number of metastatic central LNs and the LNR of metastatic LNs to the total number of retrieved LNs in patients with PTC who underwent total thyroidectomy and prophylactic CND. Prophylactic CND would be very useful to decide postoperative strategies if the information obtained after prophylactic CND such as the presence of LN metastasis, the number of positive LN, and LNR can be prognostic factors related to the recurrence.
In the current study, we found that a central LNR of 0.31, two or more metastatic central LNs, tumor size ≥20 mm and maximal ETE were independent risk factors for recurrence, whereas the presence of metastatic central LNs was not related to recurrence in multivariate analysis. The hazard ratio of LNR ≥0.31 was 11.23 (95% CI, 2.81 to 44.82), and it is higher than central LN metastasis ≥2, tumor size ≥20 mm, and maximal ETE (hazard ratio=3.43, 6.61, and 7.17, respectively). The LNR seems to be a promising prognostic factor for recurrence in patients with cN0 PTC.
The clinical significance of the number of metastatic LNs for patients with PTC remains unclear, especially in cN0 PTC. Some investigators suggested that the number of metastatic LNs is a significant predictive factor for disease recurrence [14,15]. Sugitani et al. [14] demonstrated that the risk of recurrence was significantly higher in PTC patients with five or more metastatic LNs (19%) that in those with fewer than five metastatic LNs (8%). Park et al. [15] also reported that the number of metastatic central compartment LNs (>3 metastatic LNs) in PTC was a significant predictor for recurrence conferring a hazard ratio of 1.36 (95% CI, 1.103 to 1.680). In the current study, two or more metastatic central LNs were an independent risk factor for recurrence in cN0 PTC by multivariate analysis. However, some authors have reported that the number of metastatic LNs was not associated with recurrence [21,26]. Wang et al. [26] reported that higher T stage, increased number of metastatic LNs, increased LN diameter, and presence of extranodal spread were related to recurrence in differentiated thyroid carcinoma in univariate analysis. However, in multivariate analysis, extranodal spread was the only statistically significant factor, and the number of metastatic LNs was not associated with recurrence [26]. Ryu et al. [21] also demonstrated by multivariate analysis that the number of metastatic LNs was not an independent risk factor for recurrence in pathologic N1a PTC. Further studies might be necessary to clarify the impact of number of LN metastases on the risk of recurrence in PTC considering all previous studies.
The LNR is likely to be an important prognostic factor for risk of recurrence in the previous studies. Several authors have assessed the relationship between the LNR and recurrence in patients with PTC, and reported that LNR was an important risk factor for recurrence although, in fact, the cutoff values for the LNR range from 0.26 to 0.86 in different studies [16-21,27-30]. Vas Nunes et al. [16] reported that PTC patients with an LNR of 0.30 or higher had a 3.4 times higher risk of persistent or recurrent disease compared with patients with an LNR of 0.00. In other study, LNR ≥0.86 in the central compartment had significantly worse disease-free survival rates than patients with ratios below these threshold values [17]. Jeon et al. [18] reported that LNR (higher than 0.4) and size of metastatic nodes were significant prognostic factors in pathological N1a PTC. Yip et al. [19] also demonstrated that the LNR independently predicted PTC recurrence. Schneider et al. [20] found that a LNR ≥0.42 is the best cutoff value to divide those with LN metastasis based on disease-specific survival in the study of 10,995 PTC patients utilizing the surveillance, epidemiology, and end results (SEER) database. Choi et al. [27] reported that the LNR of 0.44 is a predictive factor for the loco-regional recurrence in in patients with papillary thyroid microcarcinoma. Lee et al. [28] analyzed both central and lateral compartment LNRs: it reported that cutoff values of 0.4 and 0.5 for central LNR and total LNR, respectively, were significant risk factors for recurrence of PTC. However, Beal et al. [29] analyzed 9,926 patients with differentiated thyroid carcinoma in the SEER database, and showed that the LNR was not associated with overall survival in the pathologic N1 patients.
There are few reports that evaluated the significance of LNR in prophylactic CND for PTC. Ryu et al. [21] demonstrated that an LNR >0.65 in the central compartment was the only independent factor for recurrence in pathologic N1a PTC after prophylactic CND. Lee et al. [30] also showed that an LNR ≥0.26 was an independent predictor of regional LN metastasis recurrence in patients with PTC who underwent total thyroidectomy and bilateral prophylactic CND. In the current study, an LNR of ≥0.31 was an independent predictor of recurrence in patients with cN0 PTC who underwent total thyroidectomy and prophylactic CND.
The differences of the LNR and the number of metastatic LNs between previous studies might be related to difference in study cohorts, cutoff values for metastatic LNs and LNR, LN yields, accuracy of pathologic examination, and follow-up period. Some studies analyzed only patients with central compartment LN metastasis, whereas other workers included patients with both central and lateral LN metastasis, which might be related to more advanced disease and worse prognosis. We excluded patients who underwent concurrent lateral compartment LN dissection and included only the homogenous prophylactic CND group. The total LN yield via CND might be influenced by the extent of LN dissection and accuracy of pathologic examination. Therefore, high and low yields of LNs might be defined differently in different studies, although the LNRs might be similar. In the current study, we performed compartment-oriented CND routinely, including pretracheal, paratracheal and prelaryngeal LN groups in most patients. The mean number of harvested LNs was 7.14±6.23, which we consider to be adequate, indicating that CND was carried out correctly. We also included only those patients from whom at least two central LNs were harvested. To minimize bias and differences between studies, standardized cutoff values for the LNR and number of metastatic LNs are needed. In addition, the follow-up period differed between the studies. In some studies, the follow-up period was in the region of 5 years; however, a long-term follow-up of more than 10–20 years is necessary for accurate evaluation of recurrence and survival in patients with PTC.
There are some limitations to the current study. It is a retrospective study performed in a single center and might be influenced by a selection bias. The diverse LN yield (range, 2 to 40) during CND might also cause bias in the results, although we routinely performed compartment-oriented CND. The relatively short follow-up period is also another drawback. The mean follow-up period was 82.2±26.4 months, which might not be long enough to determine all recurrences, although most recurrences occurred within 5–10 years. A further prospective cohort study including larger numbers of homogenous patients with long-term follow up is necessary to overcome the limitations of this study.
In conclusion, tumor size ≥20 mm, maximal ETE, two or more metastatic central LNs, and an LNR ≥0.31 are independent predictors for recurrence in patients with cN0 PTC. A LNR ≥0.31 and number of metastatic LNs greater than or equal to two might be used as prognostic indicators after prophylactic CND to guide postoperative adjuvant therapy and follow-up strategy.
Go to : 
▪ This retrospective study examined the risk factors of recurrence after total thyroidectomy in 382 clinically node negative papillary thyroid carcinoma patients.
▪ Tumor size, extrathyroidal extension, number of metastatic lymph node (LN), and LN ratio (LNR) were the independent factors for recurrence.
▪ LNR ≥0.31 was associated with an 11.2-fold increased risk of post-thyroidectomy recurrence.
Go to : 
REFERENCES
1. Morris LG, Tuttle RM, Davies L. Changing trends in the incidence of thyroid cancer in the United States. JAMA Otolaryngol Head Neck Surg. 2016; Jul. 142(7):709–11.

3. Altekruse S, Das A, Cho H, Petkov V, Yu M. Do US thyroid cancer incidence rates increase with socioeconomic status among people with health insurance? An observational study using SEER population-based data. BMJ Open. 2015; Dec. 5(12):e009843.

4. Grant CS. Recurrence of papillary thyroid cancer after optimized surgery. Gland Surg. 2015; Feb. 4(1):52–62.
5. Liu FH, Kuo SF, Hsueh C, Chao TC, Lin JD. Postoperative recurrence of papillary thyroid carcinoma with lymph node metastasis. J Surg Oncol. 2015; Aug. 112(2):149–54.

6. Lan X, Sun W, Zhang H, Dong W, Wang Z, Zhang T. A meta-analysis of central lymph node metastasis for predicting lateral involvement in papillary thyroid carcinoma. Otolaryngol Head Neck Surg. 2015; Nov. 153(5):731–8.

7. Yan H, Zhou X, Jin H, Li X, Zheng M, Ming X, et al. A study on central lymph node metastasis in 543 cN0 papillary thyroid carcinoma patients. Int J Endocrinol. 2016; 2016:1878194.

8. Chereau N, Buffet C, Tresallet C, Tissier F, Leenhardt L, Menegaux F. Recurrence of papillary thyroid carcinoma with lateral cervical node metastases: predictive factors and operative management. Surgery. 2016; Mar. 159(3):755–62.

9. Park CH, Song CM, Ji YB, Pyo JY, Yi KJ, Song YS, et al. Significance of the extracapsular spread of metastatic lymph nodes in papillary thyroid carcinoma. Clin Exp Otorhinolaryngol. 2015; Sep. 8(3):289–94.

10. Ji YB, Song CM, Sung ES, Jeong JH, Lee CB, Tae K. Postoperative hypoparathyroidism and the viability of the parathyroid glands during thyroidectomy. Clin Exp Otorhinolaryngol. 2017; Sep. 10(3):265–71.

11. Ji YB, Lee DW, Song CM, Kim KR, Park CW, Tae K. Accuracy of intraoperative determination of central node metastasis by the surgeon in papillary thyroid carcinoma. Otolaryngol Head Neck Surg. 2014; Apr. 150(4):542–7.

12. Podnos YD, Smith D, Wagman LD, Ellenhorn JD. The implication of lymph node metastasis on survival in patients with well-differentiated thyroid cancer. Am Surg. 2005; Sep. 71(9):731–4.

13. Zaydfudim V, Feurer ID, Griffin MR, Phay JE. The impact of lymph node involvement on survival in patients with papillary and follicular thyroid carcinoma. Surgery. 2008; Dec. 144(6):1070–7.

14. Sugitani I, Kasai N, Fujimoto Y, Yanagisawa A. A novel classification system for patients with PTC: addition of the new variables of large (3 cm or greater) nodal metastases and reclassification during the follow-up period. Surgery. 2004; Feb. 135(2):139–48.

15. Park YM, Wang SG, Lee JC, Shin DH, Kim IJ, Son SM, et al. Metastatic lymph node status in the central compartment of papillary thyroid carcinoma: a prognostic factor of locoregional recurrence. Head Neck. 2016; Apr. 38 Suppl 1:E1172–6.

16. Vas Nunes JH, Clark JR, Gao K, Chua E, Campbell P, Niles N, et al. Prognostic implications of lymph node yield and lymph node ratio in papillary thyroid carcinoma. Thyroid. 2013; Jul. 23(7):811–6.

17. Schneider DF, Mazeh H, Chen H, Sippel RS. Lymph node ratio predicts recurrence in papillary thyroid cancer. Oncologist. 2013; 18(2):157–62.

18. Jeon MJ, Yoon JH, Han JM, Yim JH, Hong SJ, Song DE, et al. The prognostic value of the metastatic lymph node ratio and maximal metastatic tumor size in pathological N1a papillary thyroid carcinoma. Eur J Endocrinol. 2013; Jan. 168(2):219–25.

19. Yip J, Orlov S, Orlov D, Vaisman A, Hernandez KG, Etarsky D, et al. Predictive value of metastatic cervical lymph node ratio in papillary thyroid carcinoma recurrence. Head Neck. 2013; Apr. 35(4):592–8.

20. Schneider DF, Chen H, Sippel RS. Impact of lymph node ratio on survival in papillary thyroid cancer. Ann Surg Oncol. 2013; Jun. 20(6):1906–11.

21. Ryu IS, Song CI, Choi SH, Roh JL, Nam SY, Kim SY. Lymph node ratio of the central compartment is a significant predictor for locoregional recurrence after prophylactic central neck dissection in patients with thyroid papillary carcinoma. Ann Surg Oncol. 2014; Jan. 21(1):277–83.

22. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009; Nov. 19(11):1167–214.

23. Viola D, Materazzi G, Valerio L, Molinaro E, Agate L, Faviana P, et al. Prophylactic central compartment lymph node dissection in papillary thyroid carcinoma: clinical implications derived from the first prospective randomized controlled single institution study. J Clin Endocrinol Metab. 2015; Apr. 100(4):1316–24.

24. Roh JL, Park JY, Park CI. Total thyroidectomy plus neck dissection in differentiated papillary thyroid carcinoma patients: pattern of nodal metastasis, morbidity, recurrence, and postoperative levels of serum parathyroid hormone. Ann Surg. 2007; Apr. 245(4):604–10.
25. Shindo M, Wu JC, Park EE, Tanzella F. The importance of central compartment elective lymph node excision in the staging and treatment of papillary thyroid cancer. Arch Otolaryngol Head Neck Surg. 2006; Jun. 132(6):650–4.

26. Wang LY, Palmer FL, Nixon IJ, Thomas D, Shah JP, Patel SG, et al. Central lymph node characteristics predictive of outcome in patients with differentiated thyroid cancer. Thyroid. 2014; Dec. 24(12):1790–5.

27. Choi SY, Cho JK, Moon JH, Son YI. Metastatic lymph node ratio of central neck compartment has predictive values for locoregional recurrence in papillary thyroid microcarcinoma. Clin Exp Otorhinolaryngol. 2016; Mar. 9(1):75–9.

28. Lee SG, Ho J, Choi JB, Kim TH, Kim MJ, Ban EJ, et al. Optimal cut-off values of lymph node ratio predicting recurrence in papillary thyroid cancer. Medicine (Baltimore). 2016; Feb. 95(5):e2692.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download