This article has been
cited by other articles in ScienceCentral.
Abstract
Objectives
To identify the optimal pharmacological method of preparing patients for nasal endoscopy.
Methods
Twenty healthy volunteers were enrolled in this prospective, randomized, double-blind study. Four types of medications were applied in their nostrils with binary combinations of spray bottles on four different days in a random order: placebo (normal saline [NS]+NS), decongestant (NS+oxymetazoline), anesthetic (NS+lidocaine), and decongestant plus anesthetic (oxymetazoline+lidocaine). Rigid nasal endoscopy was performed 10 minutes after spray application. The volunteers evaluated the discomfort caused by each spray application, and nasal pain scores due to the passage of the endoscope. The physicians quantified nasal decongestion using a visual analogue scale. Endoscopy duration as well as pulse and mean blood pressure (MBP) before spray application, 10 minutes after the application, and immediately after endoscopic examination were also recorded.
Results
The discomfort caused by lidocaine was significantly higher than that caused by the other sprays (P<0.001). The lowest pain score related to endoscopy was obtained for oxymetazoline+lidocaine (P<0.001). Nasal decongestion was best achieved with NS+oxymetazoline (P<0.001). Endoscopy duration was the shortest for oxymetazoline+ lidocaine (P<0.05). Statistically significant MBP changes were only seen with the application of NS+oxymetazoline (P<0.05). However, neither MBP nor pulse rate change was significant clinically.
Conclusion
Application of decongestant and anesthetic sprays together seems to be the best method of pharmacological preparation of patients for nasal endoscopy.
Go to :

Keywords: Anesthetics, Nasal Decongestants, Lidocaine, Oxymetazoline, Endoscopy
INTRODUCTION
Nasal endoscopy (NE) is a widespread and valuable examination method in otolaryngology practice [
1]. NE allows the visualization of the posterior and superior parts of the nasal airway, which cannot be seen by other means, e.g., anterior rhinoscopy. NE is usually accomplished with a rigid endoscope, but a flexible endoscope is preferred in patients with severe nasal septal deviation [
2]. For the patients, NE is a painful and uncomfortable procedure [
3]; for the physicians, the disadvantages are prolonged procedure duration and limited view of nasal passages due to hypertrophic and swollen turbinates. In addition, some physiological changes such as acceleration of pulse rate and increase of blood pressure have been associated with NE [
4].
As a preparation for NE, a local anesthetic and/or a decongestant are usually applied to nostrils in order to alleviate the nasal pain, to widen the field of view, and to shorten the duration of the examination. However, the benefits of local anesthetics are controversial, partly because they cause discomfort to patients because of the bad taste and smell [
5,
6]. Furthermore, there is always a suspicion that local decongestants may cause hypertension and reflex bradycardia [
7]. Some authors recommend no medication before NE on the strength of placebo-controlled studies [
8-
11]. Other authors recommend only decongestant or decongestant–local anesthetic combination applications [
12-
14]. Nevertheless, there is no consensus about the best method of preparing the patients for NE [
1,
3,
10,
15,
16].
In this study, we aimed to identify the optimal pharmacological method of preparing the patients for NE while considering both the clinicians’ needs and the patients’ comfort and physiological stability.
Go to :

MATERIALS AND METHODS
This prospective, randomized, double-blind study was approved by the local ethics committee. After obtaining informed consents, 20 healthy volunteers were enrolled. Exclusion criteria were asthma, cardiovascular disease, rhinitis, severe septal deviation, and a history of nasal endoscopic examination.
Four spray bottles of similar external appearance were prepared and numbered; two of the bottles contained normal saline (NS; 0.9% sodium chloride), one contained Iliadin (0.05% oxymetazoline; Merck, Darmstadt, Germany), and one contained Xylocaine (10% lidocaine; AstraZeneca, Södertälje, Sweden). The nurse who prepared the bottles was the only person who was aware of the contents of the bottles.
Four binary combinations of sprays were applied in each subject’s nostrils in a random order on four different days. In each day, two puffs (one puff per bottle; 0.1 mg oxymetazoline for Iliadin, 20 mg lidocaine for Xylocaine) were applied to both nostrils by the same clinical nurse. All four combinations were applied to each subject to reduce the bias of subjective evaluation and eliminate individual differences. The application and evaluation processes were identical for each combination (
Fig. 1).
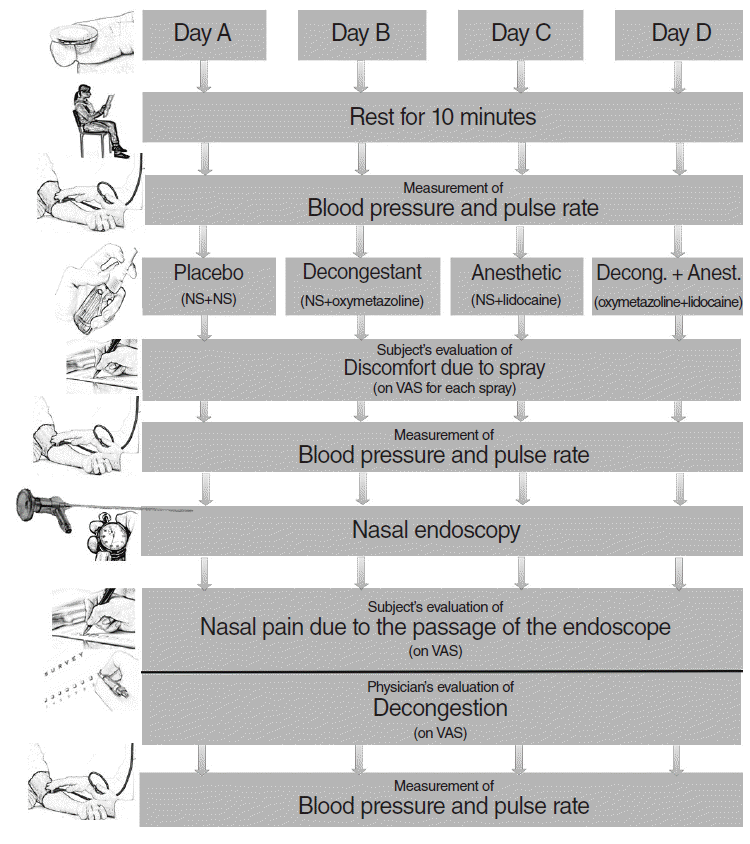 | Fig. 1.Flowchart of the method. NS, normal saline; Decong., decongestant; Anest., anesthetic; VAS, visual analog scale. 
|
Spray combinations
The four binary combinations of sprays were as follows: placebo (NS+NS), decongestant (NS+oxymetazoline), anesthetic (NS+lidocaine), decongestant plus anesthetic (oxymetazoline+lidocaine).
Nasal endoscopy
All endoscopies were performed 10 minutes after medications by the same experienced physician who was blinded to the administered drugs using a 4.0-mm, 0-degree rigid endoscope (Karl STORZ GmbH & Co. KG, Tuttlingen, Germany). In each endoscopy, choana, middle meatus, sphenoethmoidal recess, and superior concha were observed in both nasal passages. The nurse recorded the durations of the endoscopic examinations as seconds.
Clinical evaluations
Evaluation of discomfort due to the sprays
Shortly after spraying each bottle, subjects were asked to indicate their degree of discomfort on a visual analogue scale (VAS) (1: no discomfort, 10: utmost discomfort). For each combination, two independent scores were obtained from each subject (
Fig. 2).
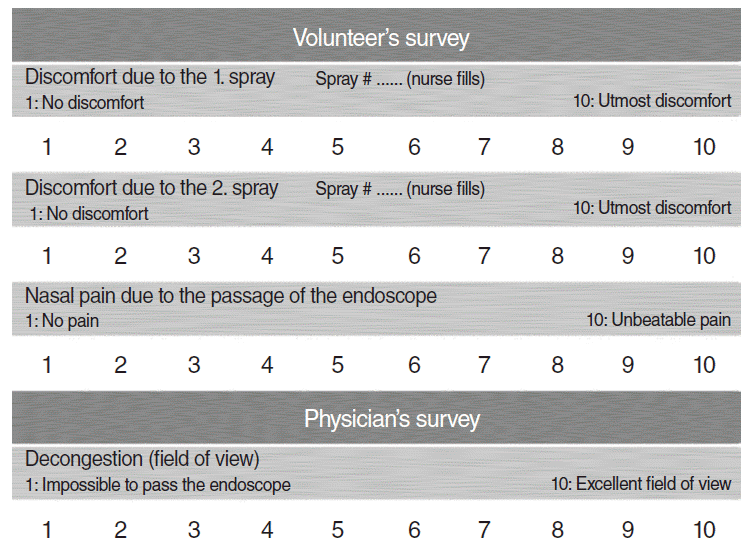 | Fig. 2.Visual analog scale (VAS) questionnaires. 
|
Evaluation of nasal pain due to the passage of the endoscope
Shortly after endoscopy, subjects were asked to indicate their degree of pain on a VAS (1: no pain, 10: unbearable pain) (
Fig. 2).
Evaluation of decongestion (field of view)
Shortly after endoscopy, the endoscopist was asked to indicate the degree of nasal decongestion on a VAS (1: impossible to pass the endoscope, 10: excellent field of view) (
Fig. 2).
Measurement of blood pressure and pulse
The blood pressure and pulse were measured thrice at each application: just before spray application (10 minutes after a rest); 10 minutes after spray application (just before the endoscopy); and immediately after the endoscopy. The same nurse did all the measurements. Blood pressure was measured with the same calibrated manometer and from the same arm of each subject. Mean blood pressures (MBP) in mmHg and change between the measurements for each application were recorded. Pulse rate was detected by palpating the radial artery, and recorded as beats per minute (BPM).
Statistical analysis
For statistical analyses, SigmaStat 3.5 (Systat Software, Inc.;
http://www.sigmaplot.co.uk/) software was used. A Shapiro-Wilk test was used to test the normality of data distribution. Normally distributed data are presented as means±standard deviation (SD). An analysis of variance test was used for repeated measures. Pairwise comparisons were performed by using the post-hoc parametric Student-Newman-Keuls method. The data, which was not normally distributed, are presented as medians (range), and a Friedman test was used for their analysis. Pairwise comparisons were performed by the nonparametric Student-Newman-Keuls method. A
P-value less than 0.05 was considered statistically significant.
Go to :

RESULTS
Twenty volunteers (12 men, 8 women) aged 27±7 years (range, 19 to 50 years) were enrolled in this study.
The discomfort caused by lidocaine was significantly higher than that caused by the other sprays (
P<0.001). There were no differences in discomfort between oxymetazoline and NS (
P>0.05) (
Fig. 3).
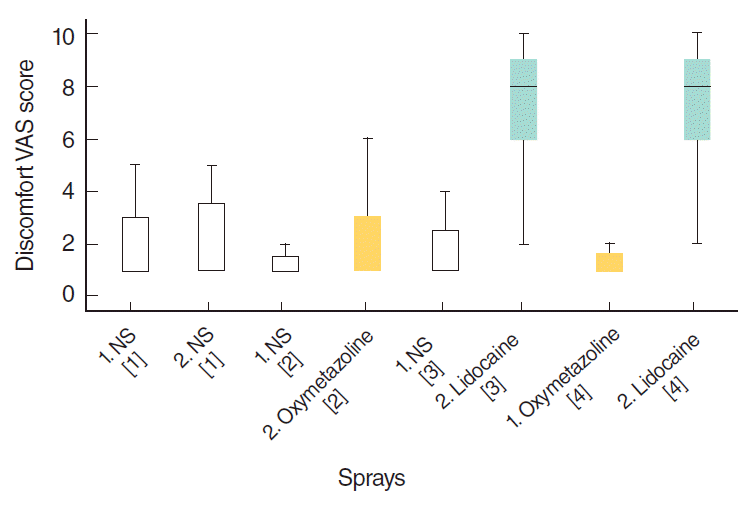 | Fig. 3.Distribution of visual analog scale (VAS) scores of discomfort due to the spray. Numbers in brackets show the type of the spray combination. [1] placebo; [2] decongestant; [3] anesthetic; [4] decongestant plus anesthetic. Numbers before the names of the sprays show the order in that combination. NS, normal saline. 
|
Nasal pain scores due to the passage of the endoscope were significantly different between spray combinations (
P<0.001). The lowest pain score was obtained for oxymetazoline+lidocaine (
P<0.05) (
Fig. 4). There were no differences in pain between NS+lidocaine and NS+NS (
P>0.05).
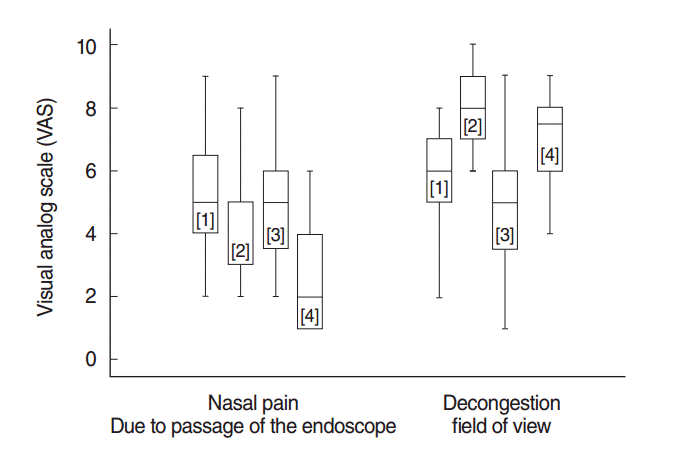 | Fig. 4.Distribution of visual analog scale (VAS) scores of nasal pain and decongestion. Numbers in brackets show the type of the spray combination. [1] placebo (NS+NS); [2] decongestant (NS+oxymeta-zoline); [3] anesthetic (NS+lidocaine); [4] decongestant plus anesthetic (oxymetazoline+lidocaine). NS, normal saline. 
|
Nasal decongestion scores were significantly different between spray combinations (
P<0.001). Nasal decongestion was best achieved with NS+oxymetazoline (
P<0.001), whereas NS+lidocaine had the least effect (
Fig. 4). Nasal decongestion scores were significantly higher for NS+oxymetazoline than for oxymetazoline+lidocaine (
P<0.05) (
Fig. 4).
Duration of endoscopy was significantly shorter for oxymetazoline+lidocaine compared to the other spray combinations (
P<0.05), and there were no differences between the other combinations (
P>0.05) (
Fig. 5).
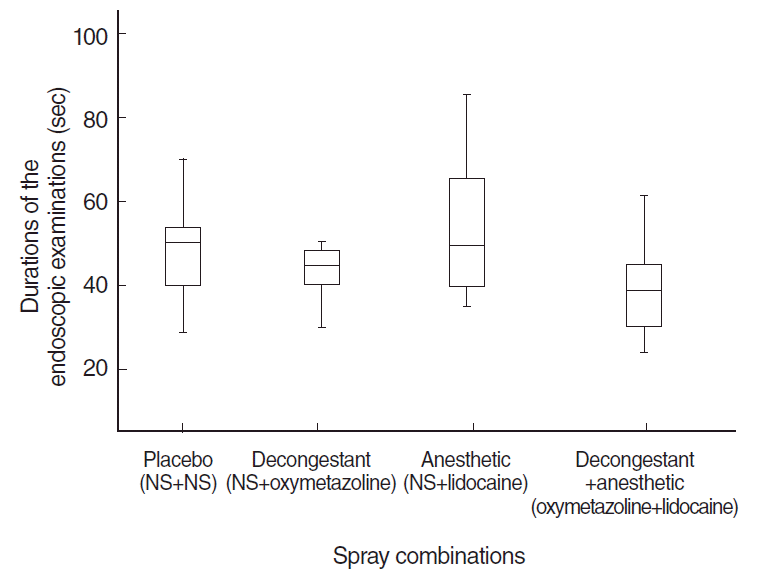 | Fig. 5.Distribution of durations of the endoscopic examinations. NS, normal saline. 
|
MBP values were increased significantly by 4.05±3.31 mmHg after NS+oxymetazoline application (
P<0.05) (
Fig. 6). However, neither this change nor the changes of MBP with the other combinations were clinically meaningful. There were no significant changes in pulse rates, neither statistically nor clinically (
P>0.05) (
Fig. 7). After endoscopy, MBP values were decreased significantly by 7.05±6.41 mmHg, and pulse rates were decreased significantly by 8.65±7.82 BPM for NS+oxymetazoline (
P<0.001). There were no differences in MBP or pulse rate for the other spray combinations (
Figs. 6,
7).
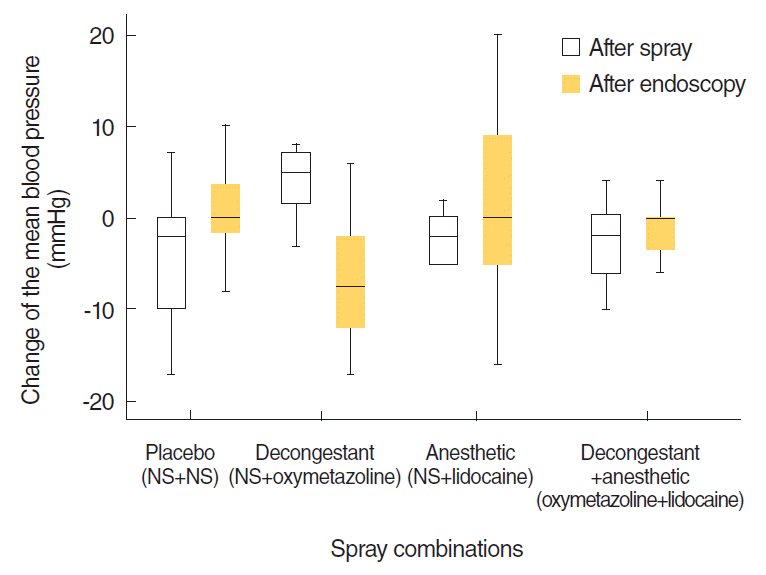 | Fig. 6.Distribution of changes of the mean blood pressure from the beginning to 10 minutes after spray application and from that point to just after endoscopy. NS, normal saline. 
|
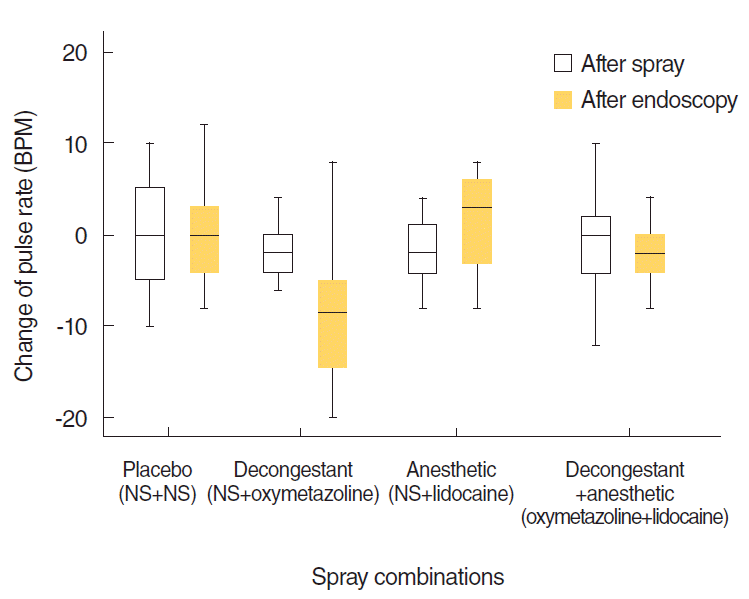 | Fig. 7.Distribution of changes of the pulse rate from the beginning to 10 minutes after spray application and from that point to just after endoscopy. NS, normal saline; BPM, beats per minute. 
|
Go to :

DISCUSSION
In this study, we compared the effects of four different spray combinations as premedication in nasal endoscopy. We confirmed that lidocaine spray is very uncomfortable for patients. Besides, it does not reduce pain during endoscopy, and it narrows the field of view in nasal passages and prolongs the duration of endoscopy. According to our results, oxymetazoline is comfortable for patients. It reduces pain during endoscopy and provides a wide field of view for the endoscopist. However, it causes a slight increase in arterial blood pressure. When oxymetazoline and lidocaine are used together, pain due to endoscopy is reduced significantly, the endoscopist’s field of view is wide, and the duration of endoscopy is shortened.
Premedication in NE is typically applied with sprays or cotton pledgets. Although application of cotton pledgets is an effective method, it is irritating for patients [
17,
18]. Thus, sprays are used more often, and we preferred sprays to cotton pledgets in this study.
It is well known that lidocaine sprays have bad taste and smell, and that they anesthetize not only the nose but also the palate, nasopharynx, and throat. These features make lidocaine sprays uncomfortable for the patients [
5,
6]. On the other hand, decongestant sprays do not have these unfavorable effects [
11]. Indeed, in this study, no differences were observed between oxymetazoline and placebo in terms of discomfort.
Interestingly, even though lidocaine is an anesthetic, it does not reduce pain during endoscopy [
5,
11,
19,
20]. In this study, we showed that pain scores were similar between lidocaine and placebo. In our opinion, the most important reason of pain during endoscopy with lidocaine is the congestion of turbinates. We reached this opinion with the evaluation of the physician’s field of view. The field of view with lidocaine was narrower than with placebo. Moreover, while nasal decongestion was best achieved with oxymetazoline, the field of view decreased when oxymetazoline was used in combination with lidocaine. On the other hand, the best pain score was attained by lidocaine plus oxymetazoline. Therefore, the combined use of decongestant and lidocaine decreased pain compared to decongestant only. Therefore, the anesthetic effect of lidocaine could be obtained when turbinates are decongested.
Some physicians avoid using premedication for NE because of its systemic effects. Even though clinicians suppose that topical decongestants cause hypertension and reflex bradycardia, there are not many studies supporting this [
7]. Similarly, NE may also increase pulse rate and arterial blood pressure [
4]. However, as clinicians, we use decongestant sprays and perform endoscopies relatively often, but do not observe these changes as often as supposed. In this study, we showed that oxymetazoline slightly increased MBP 10 minutes after its application, but MBP returned to its initial level immediately after endoscopy. Interestingly, this effect of oxymetazoline was not seen when it was combined with lidocaine. We also showed that the pulse rate did not change significantly with any of the sprays used in this study. Furthermore, the changes of pulse rate and blood pressure due to the sprays and endoscopy were not clinically relevant. However, this finding may be a result of the exclusion of elders and patients with cardiovascular diseases. Therefore, we recommend using decongestants carefully in elderly patients and in patients with cardiovascular diseases. Nonetheless, these sprays can be used safely in young and healthy individuals.
The duration of endoscopic examination can also be an issue for physicians. In this regard, we evaluated the duration of endoscopy in order to consider the demands of physicians as well as of patients. This has not been considered in any of the similar studies so far. Our findings showed that the shortest duration of examination as well as the minimum pain score were provided by the decongestant–anesthetic combination. On the other hand, the best decongestion was obtained with decongestant alone. Standing on these observations, we suggest that duration of endoscopy is not only related to the wide passage in the nose but also strongly related to the patient’s feeling of nasal pain.
In conclusion, the combined use of a decongestant and an anesthetic spray provided better field of view, reduced pain significantly, and decreased the duration of endoscopy. For these reasons, we considered decongestant plus anesthetic the best premedication method for nasal endoscopy. Because anesthetic sprays have bad taste and smell, we recommend using a decongestant spray alone in patients who refuse to use an anesthetic spray, while considering its side effects.
Go to :

HIGHLIGHTS
▪ Lidocaine discomforted the patients more than placebo.
▪ Oxymetazoline caused the best and lidocaine caused the worst decongestion score.
▪ The lowest pain score related to endoscopy was obtained for oxymetazoline plus lidocaine.
▪ Decongestant plus anesthetic spray seems to be the best premedication in nasal endoscopy.
Go to :

Notes
Go to :

ACKNOWLEDGMENTS
We thank Gülhan Şimşek, the nurse of outpatient otolaryngology clinic of Erciyes University Medical School, for helping us collecting the data. This study was presented at International Otorhinolaryngology Head & Neck Surgery Congress in Ankara, Turkey (April 17–19, 2014).
Go to :

REFERENCES
1. Gaviola GC, Chen V, Chia SH. A prospective, randomized, double-blind study comparing the efficacy of topical anesthetics in nasal endoscopy. Laryngoscope. 2013; Apr. 123(4):852–8.

2. Midwinter KI, Ahmed A, Willatt D. A randomised trial of flexible versus rigid nasendoscopy in outpatient sinonasal examination. Clin Otolaryngol Allied Sci. 2001; Aug. 26(4):281–3.

3. Nankivell PC, Pothier DD. Nasal and instrument preparation prior to rigid and flexible nasendoscopy: a systematic review. J Laryngol Otol. 2008; Oct. 122(10):1024–8.

4. Ongkasuwan J, Yung KC, Courey MS. The physiologic impact of transnasal flexible endoscopy. Laryngoscope. 2012; Jun. 122(6):1331–4.

5. Frosh AC, Jayaraj S, Porter G, Almeyda J. Is local anaesthesia actually beneficial in flexible fibreoptic nasendoscopy? Clin Otolaryngol Allied Sci. 1998; Jun. 23(3):259–62.

6. Singh V, Brockbank MJ, Todd GB. Flexible transnasal endoscopy: is local anaesthetic necessary? J Laryngol Otol. 1997; Jul. 111(7):616–8.

7. Latham GJ, Jardine DS. Oxymetazoline and hypertensive crisis in a child: can we prevent it? Paediatr Anaesth. 2013; Oct. 23(10):952–6.

8. Cain AJ, Murray DP, McClymont LG. The use of topical nasal anaesthesia before flexible nasendoscopy: a double-blind, randomized controlled trial comparing cophenylcaine with placebo. Clin Otolaryngol Allied Sci. 2002; Dec. 27(6):485–8.

9. Georgalas C, Sandhu G, Frosh A, Xenellis J. Cophenylcaine spray vs. placebo in flexible nasendoscopy: a prospective double-blind randomised controlled trial. Int J Clin Pract. 2005; Feb. 59(2):130–3.

10. Leder SB, Ross DA, Briskin KB, Sasaki CT. A prospective, double-blind, randomized study on the use of a topical anesthetic, vasoconstrictor, and placebo during transnasal flexible fiberoptic endoscopy. J Speech Lang Hear Res. 1997; Dec. 40(6):1352–7.

11. Shrestha BL, Amatya RC. Local anesthesia versus placebo in flexible nasopharyngo-laryngoscopy. J Rhinol-Otol. 2013; 1:52–6.

12. Douglas R, Hawke L, Wormald PJ. Topical anaesthesia before nasendoscopy: a randomized controlled trial of co-phenylcaine compared with lignocaine. Clin Otolaryngol. 2006; Feb. 31(1):33–5.

13. Johnson PE, Belafsky PC, Postma GN. Topical nasal anesthesia for transnasal fiberoptic laryngoscopy: a prospective, double-blind, cross-over study. Otolaryngol Head Neck Surg. 2003; Apr. 128(4):452–4.

14. Kasemsuwan L, Griffiths MV. Lignocaine with adrenaline: is it as effective as cocaine in rhinological practice? Clin Otolaryngol Allied Sci. 1996; Apr. 21(2):127–9.

15. De Freitas RP, Hannah BC. Re: nasal and instrument preparation prior to rigid and flexible nasendoscopy: a systematic review. J Laryngol Otol. 2009; Oct. 123(10):1188.
16. Sunkaraneni VS, Jones SE. Topical anaesthetic or vasoconstrictor preparations for flexible fibre-optic nasal pharyngoscopy and laryngoscopy. Cochrane Database Syst Rev. 2011; Mar. (3):CD005606.

17. Hu CT. Gauze pledgetting versus endoscopic-guided aerosolized spray for nasal anesthesia before transnasal EGD: a prospective, randomized study. Gastrointest Endosc. 2010; Jan. 71(1):11–20.

18. Mishra P, Kaushik M, Dehadaray A, Qadri H, Raichurkar A, Seth T. Preparation of nose for nasal endoscopy: cotton pledget packing versus topical spray. A prospective randomized blinded study. Eur Arch Otorhinolaryngol. 2013; Jan. 270(1):117–21.

19. Bonaparte JP, Javidnia H, Kilty S. A double-blind randomised controlled trial assessing the efficacy of topical lidocaine in extended flexible endoscopic nasal examinations. Clin Otolaryngol. 2011; Dec. 36(6):550–7.

20. Jonas NE, Visser MF, Oomen A, Albertyn R, van Dijk M, Prescott CA. Is topical local anaesthesia necessary when performing paediatric flexible nasendoscopy? A double-blind randomized controlled trial. Int J Pediatr Otorhinolaryngol. 2007; Nov. 71(11):1687–92.

Go to :







 PDF
PDF Citation
Citation Print
Print







 XML Download
XML Download