1. Kwong KK. Prevention and treatment of oropharyngeal mucositis following cancer therapy: are there new approaches. Cancer Nurs. 2004; May-Jun. 27(3):183–205.
2. Weisdorf DJ, Bostrom B, Raether D, Mattingly M, Walker P, Pihlstrom B, et al. Oropharyngeal mucositis complicating bone marrow transplantation: prognostic factors and the effect of chlorhexidine mouth rinse. Bone Marrow Transplant. 1989; Jan. 4(1):89–95.
3. Sonis ST. Mucositis: the impact, biology and therapeutic opportunities of oral mucositis. Oral Oncol. 2009; Dec. 45(12):1015–20.

4. Scully C, Epstein J, Sonis S. Oral mucositis: a challenging complication of radiotherapy, chemotherapy, and radiochemotherapy: part 1, pathogenesis and prophylaxis of mucositis. Head Neck. 2003; Dec. 25(12):1057–70.

5. Ahmed KM. The effect of olive leaf extract in decreasing the expression of two pro-inflammatory cytokines in patients receiving chemotherapy for cancer: a randomized clinical trial. Saudi Dent J. 2013; Oct. 25(4):141–7.

6. Mardani M, Afra SM, Tanideh N, Tadbir AA, Modarresi F, Koohi-Hosseinabadi O, et al. Hydroalcoholic extract of Carum carvi L. in oral mucositis: a clinical trial in male golden hamsters. Oral Dis. 2016; Jan. 22(1):39–45.
7. de Araujo RF Jr, Reinaldo MP, Brito GA, Cavalcanti Pde F, Freire MA, de Medeiros CA, et al. Olmesartan decreased levels of IL-1β and TNF-α, down-regulated MMP-2, MMP-9, COX-2, RANK/RANKL and up-regulated SOCs-1 in an intestinal mucositis model. PLoS One. 2014; Dec. 9(12):e114923.

8. Vokurka S. Oropharyngeal mucositis: pain management. Klin Onkol. 2011; 24(4):278–80.
9. Epstein JB, Tsang AH, Warkentin D, Ship JA. The role of salivary function in modulating chemotherapy-induced oropharyngeal mucositis: a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002; Jul. 94(1):39–44.

10. Brown CG, Yoder LH. Stomatitis: an overview: protecting the oral cavity during cancer treatment. Am J Nurs. 2002; Apr. 102 Suppl 4:20–3.
11. Altindag O, Kucukoglu B. Intoxication due to high dose methotrexate in a patient with rheumatoid arthritis: a case report. Turk J Rheumatol. 2011; 26(1):58–60.

12. Vanhoecke B, Bateman E, Mayo B, Vanlancker E, Stringer A, Thorpe D, et al. Dark Agouti rat model of chemotherapy-induced mucositis: establishment and current state of the art. Exp Biol Med (Maywood). 2015; Jun. 240(6):725–41.

13. Ilgenli T, Oren H, Uysal K. The acute effects of chemotherapy upon the oral cavity: prevention and management. Turk J Cancer. 2001; Jul. 31(3):93–105.
14. Jahovic N, Sener G, Cevik H, Ersoy Y, Arbak S, Yegen BC. Amelioration of methotrexate-induced enteritis by melatonin in rats. Cell Biochem Funct. 2004; May-Jun. 22(3):169–78.

15. Alamir I, Boukhettala N, Aziz M, Breuille D, Dechelotte P, Coeffier M. Beneficial effects of cathepsin inhibition to prevent chemotherapy-induced intestinal mucositis. Clin Exp Immunol. 2010; Nov. 162(2):298–305.

16. Suleyman H, Demirezer LO, Buyukokuroglu ME, Akcay MF, Gepdiremen A, Banoglu ZN, et al. Antiulcerogenic effect of Hippophae rhamnoides L. Phytother Res. 2001; Nov. 15(7):625–7.
17. Andersson SC, Rumpunen K, Johansson E, Olsson ME. Tocopherols and tocotrienols in sea buckthorn (Hippophae rhamnoides L.) berries during ripening. J Agric Food Chem. 2008; Aug. 56(15):6701–6.

18. Kwon DJ, Bae YS, Ju SM, Goh AR, Choi SY, Park J. Casuarinin suppresses TNF-α-induced ICAM-1 expression via blockade of NF-κB activation in HaCaT cells. Biochem Biophys Res Commun. 2011; Jun. 409(4):780–5.

19. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; Jun. 95(2):351–8.

20. Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968; Oct. 24. 25(1):192–205.

21. Kisaoglu A, Borekci B, Yapca OE, Bilen H, Suleyman H. Tissue damage and oxidant/antioxidant balance. Eurasian J Med. 2013; Feb. 45(1):47–9.

22. Clarkson PM, Thompson HS. Antioxidants: what role do they play in physical activity and health? Am J Clin Nutr. 2000; Aug. 72(2 Suppl):637S–46S.

23. Yeum KJ, Russell RM, Krinsky NI, Aldini G. Biomarkers of antioxidant capacity in the hydrophilic and lipophilic compartments of human plasma. Arch Biochem Biophys. 2004; Oct. 430(1):97–103.

24. Aguilar A, Alvarez-Vijande R, Capdevila S, Alcoberro J, Alcaraz A. Antioxidant patterns (superoxide dismutase, glutathione reductase, and glutathione peroxidase) in kidneys from non-heart-beating-donors: experimental study. Transplant Proc. 2007; Jan-Feb. 39(1):249–52.

25. Cetinkaya A, Bulbuloglu E, Kurutas EB, Kantarceken B. N-acetylcysteine ameliorates methotrexate-induced oxidative liver damage in rats. Med Sci Monit. 2006; Aug. 12(8):BR274–8.
26. Arslan A, Ozcicek F, Keskin Cimen F, Altuner D, Yarali O, Kurt N, et al. Protective effect of resveratrol against methotrexate-induced oxidative stress in the small intestinal tissues of rats. Int J Clin Exp Med. 2015; Jul. 8(7):10491–500.
27. Logan RM, Stringer AM, Bowen JM, Gibson RJ, Sonis ST, Keefe DM. Is the pathobiology of chemotherapy-induced alimentary tract mucositis influenced by the type of mucotoxic drug administered. Cancer Chemother Pharmacol. 2009; Jan. 63(2):239–51.

28. de Araujo AA, Borba PB, de Souza FH, Nogueira AC, Saldanha TS, Araujo TE, et al. In a methotrexate-induced model of intestinal mucositis, olmesartan reduced inflammation and induced enteropathy characterized by severe diarrhea, weight loss, and reduced sucrose activity. Biol Pharm Bull. 2015; 38(5):746–52.
29. Mills EE. The modifying effect of beta-carotene on radiation and chemotherapy induced oral mucositis. Br J Cancer. 1988; Apr. 57(4):416–7.

30. Yilmaz I, Demiryilmaz I, Sener E, Cetin N, Ucuncu Y, Altuner D, et al. The effect of hippophae rhamnoides extract on oxidative damage on rat’s gastric tissue depending on co-implementation of methotrexate and indomethacin. Lat Am J Pharm. 2014; 33(3):453–8.
31. Suryakumar G, Gupta A. Medicinal and therapeutic potential of Sea buckthorn (Hippophae rhamnoides L.). J Ethnopharmacol. 2011; Nov. 138(2):268–78.





 PDF
PDF Citation
Citation Print
Print


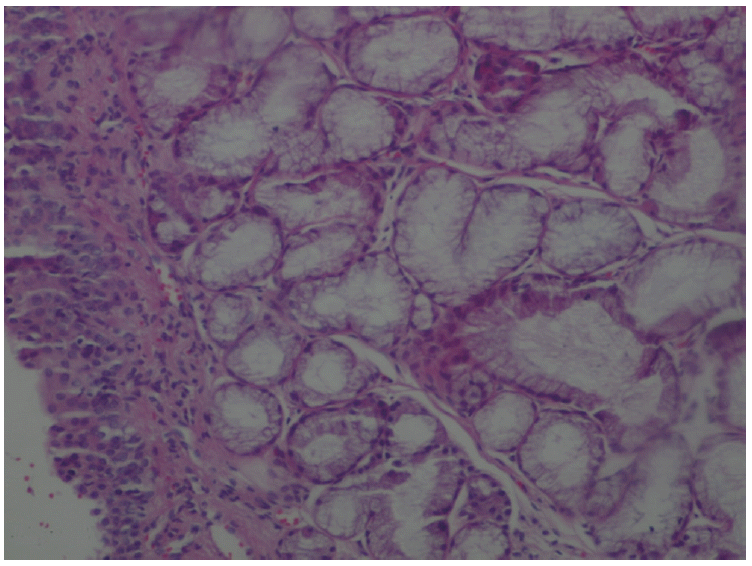
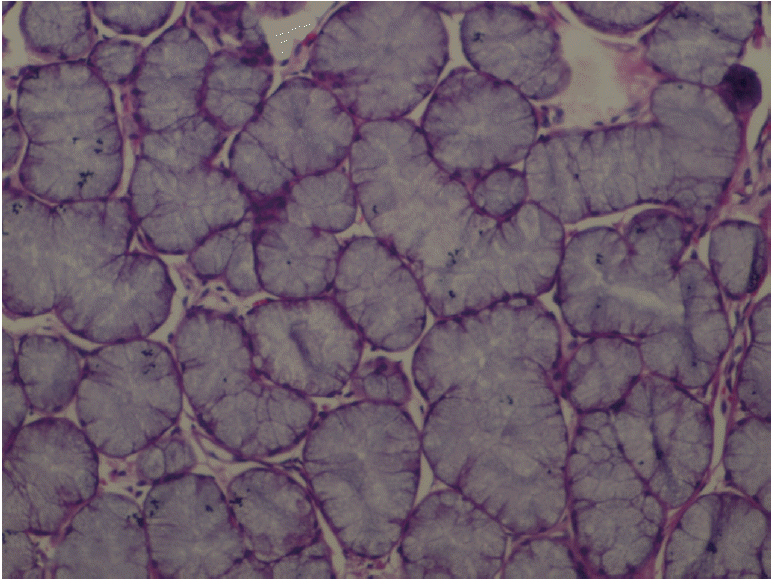
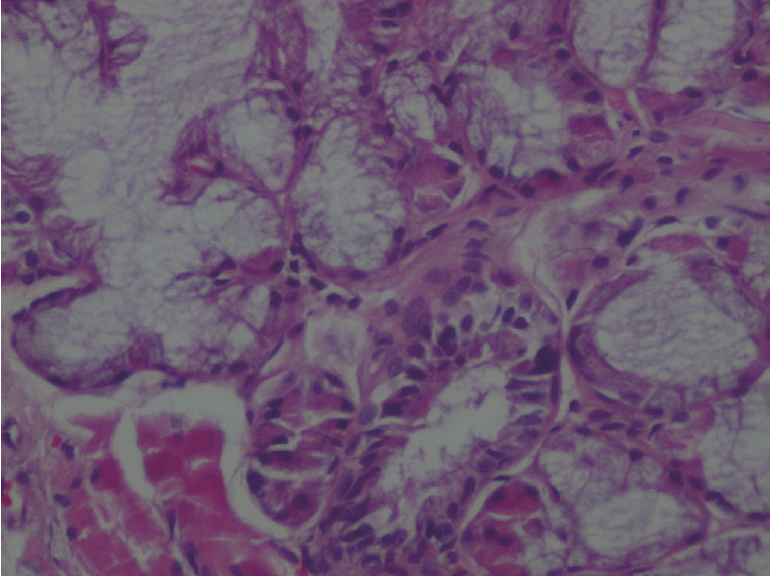
 XML Download
XML Download